Therapeutic Approaches for Preserving or Restoring Pancreatic β-Cell Function and Mass
- PMID: 25541605
- PMCID: PMC4273028
- DOI: 10.4093/dmj.2014.38.6.426
Therapeutic Approaches for Preserving or Restoring Pancreatic β-Cell Function and Mass
Abstract
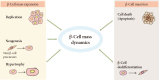
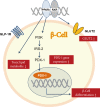
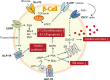
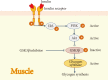
The goal for the treatment of patients with diabetes has today shifted from merely reducing glucose concentrations to preventing the natural decline in β-cell function and delay the progression of disease. Pancreatic β-cell dysfunction and decreased β-cell mass are crucial in the development of diabetes. The β-cell defects are the main pathogenesis in patients with type 1 diabetes and are associated with type 2 diabetes as the disease progresses. Recent studies suggest that human pancreatic β-cells have a capacity for increased proliferation according to increased demands for insulin. In humans, β-cell mass has been shown to increase in patients showing insulin-resistance states such as obesity or in pregnancy. This capacity might be useful for identifying new therapeutic strategies to reestablish a functional β-cell mass. In this context, therapeutic approaches designed to increase β-cell mass might prove a significant way to manage diabetes and prevent its progression. This review describes the various β-cell defects that appear in patients with diabetes and outline the mechanisms of β-cell failure. We also review common methods for assessing β-cell function and mass and methodological limitations in vivo. Finally, we discuss the current therapeutic approaches to improve β-cell function and increase β-cell mass.
Keywords: Therapeutic agents; β-Cell function; β-Cell mass.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. - PubMed
-
- Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Beta-cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90:493–500. - PubMed
-
- Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):32–42. - PubMed
-
- Peshavaria M, Larmie BL, Lausier J, Satish B, Habibovic A, Roskens V, Larock K, Everill B, Leahy JL, Jetton TL. Regulation of pancreatic beta-cell regeneration in the normoglycemic 60% partial-pancreatectomy mouse. Diabetes. 2006;55:3289–3298. - PubMed
-
- Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res. 1997;29:301–307. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

