SAFETY AND TOLERABILITY OF MRI-GUIDED INFUSION OF AAV2-hAADC INTO THE MID-BRAIN OF NON-HUMAN PRIMATE
- PMID: 25541617
- PMCID: PMC4274790
- DOI: 10.1038/mtm.2014.49
SAFETY AND TOLERABILITY OF MRI-GUIDED INFUSION OF AAV2-hAADC INTO THE MID-BRAIN OF NON-HUMAN PRIMATE
Abstract
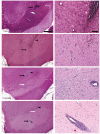
Aromatic L-amino acid decarboxylase (AADC) deficiency is a rare, autosomal-recessive neurological disorder caused by mutations in the DDC gene that leads to an inability to synthesize catecholamines and serotonin. As a result, patients suffer compromised development, particularly in motor function. A recent gene replacement clinical trial explored putaminal delivery of recombinant adeno-associated virus serotype 2 vector encoding human AADC (AAV2-hAADC) in AADC-deficient children. Unfortunately, patients presented only modest amelioration of motor symptoms, which authors acknowledged could be due to insufficient transduction of putamen. We hypothesize that, with the development of a highly accurate MRI-guided cannula placement technology, a more effective approach might be to target the affected mid-brain neurons directly. Transduction of AADC-deficient dopaminergic neurons in the substantia nigra and ventral tegmental area with locally infused AAV2-hAADC would be expected to lead to restoration of normal dopamine levels in affected children. The objective of this study was to assess the long-term safety and tolerability of bilateral AAV2-hAADC MRI-guided pressurized infusion into the mid-brain of non-human primates. Animals received either vehicle, low or high AAV2-hAADC vector dose and were euthanized 1, 3 or 9 months after surgery. Our data indicate that effective mid-brain transduction was achieved without untoward effects.
Keywords: AADC deficiency; AAV2-hAADC; MRI-guided; pressurized infusion.
Figures



Similar articles
-
Gene therapy for aromatic L-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons.Nat Commun. 2021 Jul 12;12(1):4251. doi: 10.1038/s41467-021-24524-8. Nat Commun. 2021. PMID: 34253733 Free PMC article. Clinical Trial.
-
Carbidopa-based modulation of the functional effect of the AAV2-hAADC gene therapy in 6-OHDA lesioned rats.PLoS One. 2015 Apr 10;10(4):e0122708. doi: 10.1371/journal.pone.0122708. eCollection 2015. PLoS One. 2015. PMID: 25860990 Free PMC article.
-
Safety and tolerability of magnetic resonance imaging-guided convection-enhanced delivery of AAV2-hAADC with a novel delivery platform in nonhuman primate striatum.Hum Gene Ther. 2012 Feb;23(2):210-7. doi: 10.1089/hum.2011.162. Epub 2012 Jan 26. Hum Gene Ther. 2012. PMID: 22017504 Free PMC article.
-
AADC deficiency: occurring in humans, modeled in rodents.Adv Pharmacol. 2013;68:273-84. doi: 10.1016/B978-0-12-411512-5.00013-0. Adv Pharmacol. 2013. PMID: 24054149 Review.
-
Genes for human catecholamine-synthesizing enzymes.Neurosci Res. 1991 Oct;12(2):315-45. doi: 10.1016/0168-0102(91)90001-f. Neurosci Res. 1991. PMID: 1684650 Review.
Cited by
-
Widespread AAV1- and AAV2-mediated transgene expression in the nonhuman primate brain: implications for Huntington's disease.Mol Ther Methods Clin Dev. 2016 Jun 29;3:16037. doi: 10.1038/mtm.2016.37. eCollection 2016. Mol Ther Methods Clin Dev. 2016. PMID: 27408903 Free PMC article.
-
Gene therapy restores dopamine transporter expression and ameliorates pathology in iPSC and mouse models of infantile parkinsonism.Sci Transl Med. 2021 May 19;13(594):eaaw1564. doi: 10.1126/scitranslmed.aaw1564. Sci Transl Med. 2021. PMID: 34011628 Free PMC article.
-
Improved Delivery Methods for Gene Therapy and Cell Transplantation in Parkinson's Disease.J Parkinsons Dis. 2021;11(s2):S199-S206. doi: 10.3233/JPD-212710. J Parkinsons Dis. 2021. PMID: 34366372 Free PMC article. Review.
-
Gene therapy for aromatic L-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons.Nat Commun. 2021 Jul 12;12(1):4251. doi: 10.1038/s41467-021-24524-8. Nat Commun. 2021. PMID: 34253733 Free PMC article. Clinical Trial.
-
A comparative exploration of monoamine neurotransmitter transport disorders: mechanisms, clinical manifestations, and therapeutic approaches.J Med Life. 2025 Mar;18(3):188-195. doi: 10.25122/jml-2024-0398. J Med Life. 2025. PMID: 40291937 Free PMC article. Review.
References
-
- Lee HF, Tsai CR, Chi CS, Chang TM, Lee HJ. Aromatic L-amino acid decarboxylase deficiency in Taiwan. Eur J Paediatr Neurol. 2009;13:135–140. - PubMed
-
- Manegold C, Hoffmann GF, Degen I, Ikonomidou H, Knust A, Laass MW. Aromatic L-amino acid decarboxylase deficiency: clinical features, drug therapy and follow-up. J Inherit Metab Dis. 2009;32:371–380. - PubMed
-
- Hyland K, Clayton PT. Aromatic amino acid decarboxylase deficiency in twins. J Inherit Metab Dis. 1990;13:301–304. - PubMed
-
- Brun L, Ngu LH, Keng WT, Ch’ng GS, Choy YS, Hwu WL. Clinical and biochemical features of aromatic L-amino acid decarboxylase deficiency. Neurology. 2010;75:64–71. - PubMed
-
- Hoffmann GF, Assmann B, Bräutigam C, Dionisi-Vici C, Häussler M, de Klerk JB. Tyrosine hydroxylase deficiency causes progressive encephalopathy and dopa-nonresponsive dystonia. Ann Neurol. 2003;54 (suppl. 6):S56–S65. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

