Stem cells, progenitor cells, and lineage decisions in the ovary
- PMID: 25541635
- PMCID: PMC4496428
- DOI: 10.1210/er.2014-1079
Stem cells, progenitor cells, and lineage decisions in the ovary
Abstract
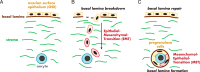
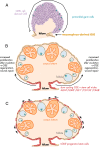
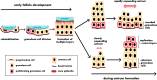
Exploring stem cells in the mammalian ovary has unleashed a Pandora's box of new insights and questions. Recent evidence supports the existence of stem cells of a number of the different cell types within the ovary. The evidence for a stem cell model producing mural granulosa cells and cumulus cells is strong, despite a limited number of reports. The recent identification of a precursor granulosa cell, the gonadal ridge epithelial-like cell, is exciting and novel. The identification of female germline (oogonial) stem cells is still very new and is currently limited to just a few species. Their origins and physiological roles, if any, are unknown, and their potential to produce oocytes and contribute to follicle formation in vivo lacks robust evidence. The precursor of thecal cells remains elusive, and more compelling data are needed. Similarly, claims of very small embryonic-like cells are also preliminary. Surface epithelial cells originating from gonadal ridge epithelial-like cells and from the mesonephric epithelium at the hilum of the ovary have also been proposed. Another important issue is the role of the stroma in guiding the formation of the ovary, ovigerous cords, follicles, and surface epithelium. Immune cells may also play key roles in developmental patterning, given their critical roles in corpora lutea formation and regression. Thus, while the cellular biology of the ovary is extremely important for its major endocrine and fertility roles, there is much still to be discovered. This review draws together the current evidence and perspectives on this topic.
Figures



References
-
- Hsueh AJ, Kawamura K, Cheng Y, Fauser BC. Intraovarian control of early folliculogenesis [published online September 9, 2014]. Endocr Rev. doi.org/10.1210/er.2014–1020. - DOI - PMC - PubMed
-
- Matsuda F, Inoue N, Manabe N, Ohkura S. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev. 2012;58:44–50. - PubMed
-
- Scaramuzzi RJ, Baird DT, Campbell BK, et al. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod Fertil Dev. 2011;23:444–467. - PubMed
-
- Chaves RN, de Matos MH, Buratini J, Jr, de Figueiredo JR. The fibroblast growth factor family: involvement in the regulation of folliculogenesis. Reprod Fertil Dev. 2012;24:905–915. - PubMed
-
- Chaves RN, Alves AM, Lima LF, Matos HM, Rodrigues AP, Figueiredo JR. Role of nerve growth factor (NGF) and its receptors in folliculogenesis. Zygote. 2013;21:187–197. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

