Evidence for the involvement of loosely bound plastosemiquinones in superoxide anion radical production in photosystem II
- PMID: 25541694
- PMCID: PMC4277363
- DOI: 10.1371/journal.pone.0115466
Evidence for the involvement of loosely bound plastosemiquinones in superoxide anion radical production in photosystem II
Erratum in
-
Correction: Evidence for the Involvement of Loosely Bound Plastosemiquinones in Superoxide Anion Radical Production in Photosystem II.PLoS One. 2015 Jun 4;10(6):e0130244. doi: 10.1371/journal.pone.0130244. eCollection 2015. PLoS One. 2015. PMID: 26043117 Free PMC article. No abstract available.
Abstract
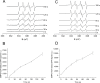
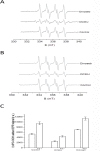
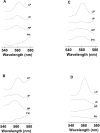
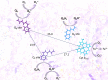
Recent evidence has indicated the presence of novel plastoquinone-binding sites, QC and QD, in photosystem II (PSII). Here, we investigated the potential involvement of loosely bound plastosemiquinones in superoxide anion radical (O2-) formation in spinach PSII membranes using electron paramagnetic resonance (EPR) spin-trapping spectroscopy. Illumination of PSII membranes in the presence of the spin trap EMPO (5-(ethoxycarbonyl)-5-methyl-1-pyrroline N-oxide) resulted in the formation of O2-, which was monitored by the appearance of EMPO-OOH adduct EPR signal. Addition of exogenous short-chain plastoquinone to PSII membranes markedly enhanced the EMPO-OOH adduct EPR signal. Both in the unsupplemented and plastoquinone-supplemented PSII membranes, the EMPO-OOH adduct EPR signal was suppressed by 50% when the urea-type herbicide DCMU (3-(3,4-dichlorophenyl)-1,1-dimethylurea) was bound at the QB site. However, the EMPO-OOH adduct EPR signal was enhanced by binding of the phenolic-type herbicide dinoseb (2,4-dinitro-6-sec-butylphenol) at the QD site. Both in the unsupplemented and plastoquinone-supplemented PSII membranes, DCMU and dinoseb inhibited photoreduction of the high-potential form of cytochrome b559 (cyt b559). Based on these results, we propose that O2- is formed via the reduction of molecular oxygen by plastosemiquinones formed through one-electron reduction of plastoquinone at the QB site and one-electron oxidation of plastoquinol by cyt b559 at the QC site. On the contrary, the involvement of a plastosemiquinone formed via the one-electron oxidation of plastoquinol by cyt b559 at the QD site seems to be ambiguous. In spite of the fact that the existence of QC and QD sites is not generally accepted yet, the present study provided more spectroscopic data on the potential functional role of these new plastoquinone-binding sites.
Conflict of interest statement
Figures

References
-
- Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S (2004) Architecture of the photosynthetic oxygen-evolving center. Science 303: 1831–1838. - PubMed
-
- Renger G, Holzwarth AR (2005) Primary electron transfer in the RC II In: Wydrzynski TJ, Satoh K (eds.), Photosystem II: the light-driven water: plastoquinoneoxidoreductase, Springer, Dordrecht, 139–175.
-
- Rappaport F, Diner BA (2008) Primary photochemistry and energetics leading to the oxidation of the (Mn)4Ca cluster and to the evolution of molecular oxygen in photosystem II. Coord Chem Rev 252: 259–272.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

