Analysis of potato virus Y coat protein epitopes recognized by three commercial monoclonal antibodies
- PMID: 25542005
- PMCID: PMC4277358
- DOI: 10.1371/journal.pone.0115766
Analysis of potato virus Y coat protein epitopes recognized by three commercial monoclonal antibodies
Abstract
Background: Potato virus Y (PVY, genus Potyvirus) causes substantial economic losses in solanaceous plants. Routine screening for PVY is an essential part of seed potato certification, and serological assays are often used. The commercial, commonly used monoclonal antibodies, MAb1128, MAb1129, and MAb1130, recognize the viral coat protein (CP) of PVY and distinguish PVYN strains from PVYO and PVYC strains, or detect all PVY strains, respectively. However, the minimal epitopes recognized by these antibodies have not been identified.
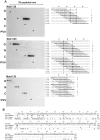
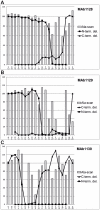
Methodology/principal findings: SPOT peptide array was used to map the epitopes in CP recognized by MAb1128, MAb1129, and MAb1130. Then alanine replacement as well as N- and C-terminal deletion analysis of the identified peptide epitopes was done to determine critical amino acids for antibody recognition and the respective minimal epitopes. The epitopes of all antibodies were located within the 30 N-terminal-most residues. The minimal epitope of MAb1128 was 25NLNKEK30. Replacement of 25N or 27N with alanine weakened the recognition by MAb1128, and replacement of 26L, 29E, or 30K nearly precluded recognition. The minimal epitope for MAb1129 was 16RPEQGSIQSNP26 and the most critical residues for recognition were 22I and 23Q. The epitope of MAb1130 was defined by residues 5IDAGGS10. Mutation of residue 6D abrogated and mutation of 9G strongly reduced recognition of the peptide by MAb1130. Amino acid sequence alignment demonstrated that these epitopes are relatively conserved among PVY strains. Finally, recombinant CPs were produced to demonstrate that mutations in the variable positions of the epitope regions can affect detection with the MAbs.
Conclusions/significance: The epitope data acquired can be compared with data on PVY CP-encoding sequences produced by laboratories worldwide and utilized to monitor how widely the new variants of PVY can be detected with current seed potato certification schemes or during the inspection of imported seed potatoes as conducted with these MAbs.
Conflict of interest statement
Figures



Similar articles
-
Characterization and strain identification of a potato virus Y isolate non-reactive with monoclonal antibodies specific to the ordinary and necrotic strains.Intervirology. 1994;37(1):12-9. doi: 10.1159/000150349. Intervirology. 1994. PMID: 7928283
-
Analysis of the epitope structure of Plum pox virus coat protein.Phytopathology. 2011 May;101(5):611-9. doi: 10.1094/PHYTO-10-10-0274. Phytopathology. 2011. PMID: 21171886
-
Recognition of Linear B-Cell Epitope of Betanodavirus Coat Protein by RG-M18 Neutralizing mAB Inhibits Giant Grouper Nervous Necrosis Virus (GGNNV) Infection.PLoS One. 2015 May 4;10(5):e0126121. doi: 10.1371/journal.pone.0126121. eCollection 2015. PLoS One. 2015. PMID: 25938761 Free PMC article.
-
Identification of three linear B cell epitopes using monoclonal antibodies against bovine enterovirus VP2 protein.Appl Microbiol Biotechnol. 2019 Sep;103(18):7467-7480. doi: 10.1007/s00253-019-09971-0. Epub 2019 Jun 28. Appl Microbiol Biotechnol. 2019. PMID: 31253999
-
The epitope structure of Citrus tristeza virus coat protein mapped by recombinant proteins and monoclonal antibodies.Virology. 2014 Jan 5;448:238-46. doi: 10.1016/j.virol.2013.10.021. Epub 2013 Oct 31. Virology. 2014. PMID: 24314654
Cited by
-
Graphical genotyping as a method to map Ny (o,n)sto and Gpa5 using a reference panel of tetraploid potato cultivars.Theor Appl Genet. 2017 Mar;130(3):515-528. doi: 10.1007/s00122-016-2831-y. Epub 2016 Nov 21. Theor Appl Genet. 2017. PMID: 27872942 Free PMC article.
-
Unleashing the power of colloidal gold immunochromatographic assays for plant virus diagnostics.MethodsX. 2023 Nov 29;12:102498. doi: 10.1016/j.mex.2023.102498. eCollection 2024 Jun. MethodsX. 2023. PMID: 38089155 Free PMC article. Review.
-
Epitope mapping and a cocktail of monoclonal antibodies to achieve full detection coverage of potato virus Y.Plant Biotechnol J. 2023 Sep;21(9):1725-1727. doi: 10.1111/pbi.14094. Epub 2023 Jun 8. Plant Biotechnol J. 2023. PMID: 37288597 Free PMC article. No abstract available.
References
-
- Adams MJ, Zerbini FM, French R, Rabenstein F, Stenger DC, et al.. (2012) Family Potyviridae. In: King AM, Adams MJ, Carstens EB and Lefkowich EJeditors. Virus Taxonomy. San Diego: Elsevier, pp. 1069–1089.
-
- Valkonen JPT (2007) Chapter 28 - Viruses: Economical losses and biotechnological potential. In: Vreugdenhil D, Bradshaw J, GebhardtC, Govers F, Mackerron DKL, Taylor M Aet al.. editors. Potato Biology and Biotechnology. Amsterdam: Elsevier Science B.V. pp. 619–641.
-
- Tian YP, Liu JL, Zhang CL, Liu YY, Wang B, et al. (2011) Genetic diversity of Potato virus Y infecting tobacco crops in China. Phytopathology 101:377–387. - PubMed
-
- Romero A, Blanco-Urgoiti B, Soto MJ, Fereres A, Ponz F (2001) Characterization of typical pepper-isolates of PVY reveals multiple pathotypes within a single genetic strain. Virus Res 79:71–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

