Characterization of primary cultures of adult human epididymis epithelial cells
- PMID: 25542823
- PMCID: PMC4346407
- DOI: 10.1016/j.fertnstert.2014.11.022
Characterization of primary cultures of adult human epididymis epithelial cells
Abstract
Objective: To establish cultures of epithelial cells from all regions of the human epididymis to provide reagents for molecular approaches to functional studies of this epithelium.
Design: Experimental laboratory study.
Setting: University research institute.
Patient(s): Epididymis from seven patients undergoing orchiectomy for suspected testicular cancer without epididymal involvement.
Intervention(s): Human epididymis epithelial cells harvested from adult epididymis tissue.
Main outcome measure(s): Establishment of a robust culture protocol for adult human epididymal epithelial cells.
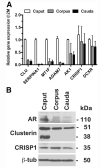
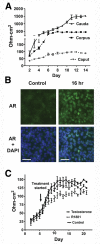
Result(s): Cultures of caput, corpus, and cauda epithelial cells were established from epididymis tissue of seven donors. Cells were passaged up to eight times and maintained differentiation markers. They were also cryopreserved and recovered successfully. Androgen receptor, clusterin, and cysteine-rich secretory protein 1 were expressed in cultured cells, as shown by means of immunofluorescence, Western blot, and quantitative reverse-transcription polymerase chain reaction (qRT-PCR). The distribution of other epididymis markers was also shown by means of qRT-PCR. Cultures developed transepithelial resistance (TER), which was androgen responsive in the caput but androgen insensitive in the corpus and cauda, where unstimulated TER values were much higher.
Conclusion(s): The results demonstrate a robust in vitro culture system for differentiated epithelial cell types in the caput, corpus, and cauda of the human epididymis. These cells will be a valuable resource for molecular analysis of epididymis epithelial function, which has a pivotal role in male fertility.
Keywords: Epididymis epithelial cell culture: caput; cauda; corpus.
Copyright © 2015 American Society for Reproductive Medicine. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Expression profiles of human epididymis epithelial cells reveal the functional diversity of caput, corpus and cauda regions.Mol Hum Reprod. 2016 Feb;22(2):69-82. doi: 10.1093/molehr/gav066. Epub 2015 Nov 26. Mol Hum Reprod. 2016. PMID: 26612782 Free PMC article.
-
Diverse secretory patterns of clusterin by epididymis and prostate/seminal vesicles undergoing cell regression after orchiectomy.Endocrinology. 1990 Jun;126(6):2989-97. doi: 10.1210/endo-126-6-2989. Endocrinology. 1990. PMID: 2351105
-
In vitro culture of epithelial cells from the caput, corpus, and cauda epididymis of Sus domesticus.Theriogenology. 2004 Sep 1;62(5):929-42. doi: 10.1016/j.theriogenology.2003.12.015. Theriogenology. 2004. PMID: 15251244
-
The expression spectrum of yak epididymal epithelial cells reveals the functional diversity of caput, corpus and cauda regions.Genomics. 2024 Sep;116(5):110912. doi: 10.1016/j.ygeno.2024.110912. Epub 2024 Aug 6. Genomics. 2024. PMID: 39117249 Review.
-
Regulation of epididymal epithelial cell functions.Biol Reprod. 1995 Feb;52(2):226-36. doi: 10.1095/biolreprod52.2.226. Biol Reprod. 1995. PMID: 7711192 Review.
Cited by
-
Morphological and morphometric changes and epithelial apoptosis are induced in the rat epididymis by long-term letrozole treatment.Eur J Histochem. 2021 Sep 3;65(3):3259. doi: 10.4081/ejh.2021.3259. Eur J Histochem. 2021. PMID: 34474552 Free PMC article.
-
HNF1 regulates critical processes in the human epididymis epithelium.Mol Cell Endocrinol. 2016 Apr 15;425:94-102. doi: 10.1016/j.mce.2016.01.021. Epub 2016 Jan 22. Mol Cell Endocrinol. 2016. PMID: 26808453 Free PMC article.
-
A transcription factor network represses CFTR gene expression in airway epithelial cells.Biochem J. 2018 Apr 16;475(7):1323-1334. doi: 10.1042/BCJ20180044. Biochem J. 2018. PMID: 29572268 Free PMC article.
-
Differential contribution of cis-regulatory elements to higher order chromatin structure and expression of the CFTR locus.Nucleic Acids Res. 2016 Apr 20;44(7):3082-94. doi: 10.1093/nar/gkv1358. Epub 2015 Dec 15. Nucleic Acids Res. 2016. PMID: 26673704 Free PMC article.
-
Cell-Selective Regulation of CFTR Gene Expression: Relevance to Gene Editing Therapeutics.Genes (Basel). 2019 Mar 19;10(3):235. doi: 10.3390/genes10030235. Genes (Basel). 2019. PMID: 30893953 Free PMC article. Review.
References
-
- Cornwall GA, von Horsten HH. Sperm Maturation in the epididymis. In: Carrell DT, editor. The genetics of male infertility. Humana Press; Totowa, New Jersey: 2007. pp. 211–31.
-
- Dacheux JL, Dacheux F. Protein secretion in the epididymis. In: Robaire B, Hinton BT, editors. The epididymis: from molecules to clinical practice. Springer; New York: 2002. pp. 151–68.
-
- Cornwall GA, Lareyre JJ, Matusik RI, Hinton BT, Orgebin-Crist MC. Gene expression and epididymal function. In: Robaire B, Hinton BT, editors. The epididymis: from molecules to clinical practice. Springer; New York: 2002. pp. 169–200.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

