Voltage-gated Ca2+ entry promotes oligodendrocyte progenitor cell maturation and myelination in vitro
- PMID: 25542980
- PMCID: PMC4711374
- DOI: 10.1016/j.expneurol.2014.12.012
Voltage-gated Ca2+ entry promotes oligodendrocyte progenitor cell maturation and myelination in vitro
Abstract
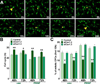
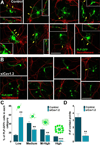
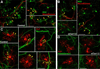
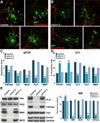
We have previously shown that the expression of voltage-operated Ca(++) channels (VOCCs) is highly regulated in the oligodendroglial lineage and is essential for proper oligodendrocyte progenitor cell (OPC) migration. Here we assessed the role of VOCCs, in particular the L-type, in oligodendrocyte maturation. We used pharmacological treatments to activate or block voltage-gated Ca(++) uptake and siRNAs to specifically knock down the L-type VOCC in primary cultures of mouse OPCs. Activation of VOCCs by plasma membrane depolarization increased OPC morphological differentiation as well as the expression of mature oligodendrocyte markers. On the contrary, inhibition of L-type Ca(++) channels significantly delayed OPC development. OPCs transfected with siRNAs for the Cav1.2 subunit that conducts L-type Ca(++) currents showed reduce Ca(++) influx by ~75% after plasma membrane depolarization, indicating that Cav1.2 is heavily involved in mediating voltage-operated Ca(++) entry in OPCs. Cav1.2 knockdown induced a decrease in the proportion of oligodendrocytes that expressed myelin proteins, and an increase in cells that retained immature oligodendrocyte markers. Moreover, OPC proliferation, but not cell viability, was negatively affected after L-type Ca(++) channel knockdown. Additionally, we have tested the ability of L-type VOCCs to facilitate axon-glial interaction during the first steps of myelin formation using an in vitro co-culture system of OPCs with cortical neurons. Unlike control OPCs, Cav1.2 deficient oligodendrocytes displayed a simple morphology, low levels of myelin proteins expression and appeared to be less capable of establishing contacts with neurites and axons. Together, this set of in vitro experiments characterizes the involvement of L-type VOCCs on OPC maturation as well as the role played by these Ca(++) channels during the early phases of myelination.
Keywords: Calcium influx; Myelination; Oligodendrocyte; Voltage-operated Ca(++) channels.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures







References
-
- Agresti C, Meomartini ME, Amadio S, Ambrosini E, Volonté C, Aloisi F, Visentin S. ATP regulates oligodendrocyte progenitor migration, proliferation, and differentiation: involvement of metabotropic P2 receptors. Brain Res Rev. 2005;48:157–165. - PubMed
-
- Agresti C, D’Urso D, Levi G. Reversible inhibitory effects of interferon-γ and tumour necrosis factor-α on oligodendroglial lineage cell proliferation and differentiation in vitro. Eur J Neurosci. 1996;8:1106–1116. - PubMed
-
- Alberdi E, Sanchez-Gomez MV, Matute C. Calcium and glial cell death. Cell Calcium. 2005;38:417–425. - PubMed
-
- Amur-Umarjee S, Phan T, Campagnoni AT. Myelin basic protein mRNA translocation in oligodendrocytes is inhibited by astrocytes in vitro. J Neurosci Res. 1993;36:99–110. - PubMed
-
- Belachew S, Malgrange B, Rigo JM, Rogister B, Leprince P, Hans G, Nguyen L, Moonen G. Glycine triggers an intracellular calcium influx in oligodendrocyte progenitor cells which is mediated by the activation of both the ionotropic glycine receptor and Na+-dependent transporters. Eur J Neurosci. 2000;12:1924–1930. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

