Low dose radiation induced senescence of human mesenchymal stromal cells and impaired the autophagy process
- PMID: 25544750
- PMCID: PMC4480742
- DOI: 10.18632/oncotarget.2692
Low dose radiation induced senescence of human mesenchymal stromal cells and impaired the autophagy process
Abstract
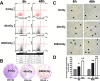
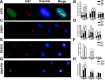
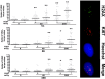
Low doses of radiation may have profound effects on cellular function. Individuals may be exposed to low doses of radiation either intentionally for medical purposes or accidentally, such as those exposed to radiological terrorism or those who live near illegal radioactive waste dumpsites.We studied the effects of low dose radiation on human bone marrow mesenchymal stromal cells (MSC), which contain a subpopulation of stem cells able to differentiate in bone, cartilage, and fat; support hematopoiesis; and contribute to body's homeostasis.The main outcome of low radiation exposure, besides reduction of cell cycling, is the triggering of senescence, while the contribution to apoptosis is minimal. We also showed that low radiation affected the autophagic flux. We hypothesize that the autophagy prevented radiation deteriorative processes, and its decline contributed to senescence.An increase in ATM staining one and six hours post-irradiation and return to basal level at 48 hours, along with persistent gamma-H2AX staining, indicated that MSC properly activated the DNA repair signaling, though some damages remained unrepaired, mainly in non-cycling cells. This suggested that the impaired DNA repair capacity of irradiated MSC seemed mainly related to the reduced activity of a non-homologous end-joining (NHEJ) system rather than HR (homologous recombination).
Keywords: mesenchymal stem cells; radiation; senescence.
Figures


References
-
- Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080–1086. - PubMed
-
- Sperka T, Wang J, Rudolph KL. DNA damage checkpoints in stem cells, ageing and cancer. Nature reviews Molecular cell biology. 2012;13:579–590. - PubMed
-
- Cmielova J, Havelek R, Soukup T, Jiroutova A, Visek B, Suchanek J, Vavrova J, Mokry J, Muthna D, Bruckova L, Filip S, English D, Rezacova M. Gamma radiation induces senescence in human adult mesenchymal stem cells from bone marrow and periodontal ligaments. International journal of radiation biology. 2012;88:393–404. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

