Functionalized fullerenes in photodynamic therapy
- PMID: 25544837
- PMCID: PMC4276409
- DOI: 10.1166/jbn.2014.1963
Functionalized fullerenes in photodynamic therapy
Abstract
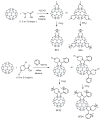
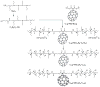
Since the discovery of C60 fullerene in 1985, scientists have been searching for biomedical applications of this most fascinating of molecules. The unique photophysical and photochemical properties of C60 suggested that the molecule would function well as a photosensitizer in photodynamic therapy (PDT). PDT uses the combination of non-toxic dyes and harmless visible light to produce reactive oxygen species that kill unwanted cells. However the extreme insolubility and hydrophobicity of pristine CO60, mandated that the cage be functionalized with chemical groups that provided water solubility and biological targeting ability. It has been found that cationic quaternary ammonium groups provide both these features, and this review covers work on the use of cationic fullerenes to mediate destruction of cancer cells and pathogenic microorganisms in vitro and describes the treatment of tumors and microbial infections in mouse models. The design, synthesis, and use of simple pyrrolidinium salts, more complex decacationic chains, and light-harvesting antennae that can be attached to C60, C70 and C84 cages are covered. In the case of bacterial wound infections mice can be saved from certain death by fullerene-mediated PDT.
Figures









References
-
- Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photobiol. 1992;55:145. - PubMed
-
- Boyle RW, Dolphin D. Structure and biodistribution relationships of photodynamic sensitizers. Photochem Photobiol. 1996;64:469. - PubMed
-
- Sternberg ED, Dolphin D, Brückner C. Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron. 1998;54:4151.
-
- Ravindra K, Pandey RK, Zheng G. In: Porphyrins as photosensitizers in photodynamic therapy, The Porphyrin Handbook. Kadish K, Guilard R, Smiths KM, editors. Vol. 6. Elsevier; San Diego: 2000. pp. 157–161.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
