C-terminal juxtamembrane region of full-length M2 protein forms a membrane surface associated amphipathic helix
- PMID: 25545360
- PMCID: PMC4353368
- DOI: 10.1002/pro.2631
C-terminal juxtamembrane region of full-length M2 protein forms a membrane surface associated amphipathic helix
Abstract
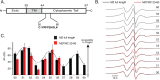
The influenza A M2 protein is a 97-residue integral membrane protein involved in viral budding and proton conductance. Although crystal and NMR structures exist of truncated constructs of the protein, there is disagreement between models and only limited structural data are available for the full-length protein. Here, the structure of the C-terminal juxtamembrane region (sites 50-60) is investigated in the full-length M2 protein using site-directed spin-labeling electron paramagnetic resonance (EPR) spectroscopy in lipid bilayers. Sites 50-60 were chosen for study because this region has been shown to be critical to the role the M2 protein plays in viral budding. Continuous wave EPR spectra and power saturation data in the presence of paramagnetic membrane soluble oxygen are consistent with a membrane surface associated amphipathic helix. Comparison between data from the C-terminal juxtamembrane region in full-length M2 protein with data from a truncated M2 construct demonstrates that the line shapes and oxygen accessibilities are remarkably similar between the full-length and truncated form of the protein.
Keywords: amphipathic helix; electron paramagnetic resonance; full-length M2 protein; site-directed spin labeling; viral budding.
© 2014 The Protein Society.
Figures
References
-
- Pinto LH, Lamb RA. The M2 proton channels of influenza A and B viruses. J Biol Chem. 2006;281:8997–9000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

