Levodopa-carbidopa intestinal gel in advanced Parkinson's disease: final 12-month, open-label results
- PMID: 25545465
- PMCID: PMC4674978
- DOI: 10.1002/mds.26123
Levodopa-carbidopa intestinal gel in advanced Parkinson's disease: final 12-month, open-label results
Abstract
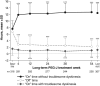
Motor complications in Parkinson's disease (PD) are associated with long-term oral levodopa treatment and linked to pulsatile dopaminergic stimulation. L-dopa-carbidopa intestinal gel (LCIG) is delivered continuously by percutaneous endoscopic gastrojejunostomy tube (PEG-J), which reduces L-dopa-plasma-level fluctuations and can translate to reduced motor complications. We present final results of the largest international, prospective, 54-week, open-label LCIG study. PD patients with severe motor fluctuations (>3 h/day "off" time) despite optimized therapy received LCIG monotherapy. Additional PD medications were allowed >28 days post-LCIG initiation. Safety was the primary endpoint measured through adverse events (AEs), device complications, and number of completers. Secondary endpoints included diary-assessed off time, "on" time with/without troublesome dyskinesia, UPDRS, and health-related quality-of-life (HRQoL) outcomes. Of 354 enrolled patients, 324 (91.5%) received PEG-J and 272 (76.8%) completed the study. Most AEs were mild/moderate and transient; complication of device insertion (34.9%) was the most common. Twenty-seven (7.6%) patients withdrew because of AEs. Serious AEs occurred in 105 (32.4%), most commonly complication of device insertion (6.5%). Mean daily off time decreased by 4.4 h/65.6% (P < 0.001). On time without troublesome dyskinesia increased by 4.8 h/62.9% (P < 0.001); on time with troublesome dyskinesia decreased by 0.4 h/22.5% (P = 0.023). Improvements persisted from week 4 through study completion. UPDRS and HRQoL outcomes were also improved throughout. In the advanced PD population, LCIG's safety profile consisted primarily of AEs associated with the device/procedure, l-dopa/carbidopa, and advanced PD. LCIG was generally well tolerated and demonstrated clinically significant improvements in motor function, daily activities, and HRQoL sustained over 54 weeks.
Trial registration: ClinicalTrials.gov NCT00335153.
Keywords: dyskinesia; infusion; levodopa-carbidopa intestinal gel; percutaneous endoscopic gastrojejunostomy; “off” time.
© 2014 The Authors. Movement Disorders published by Wiley Periodicals, Inc. on behalf of International Parkinson and Movement Disorder Society.
Figures


Comment in
-
Continuous levodopa infusion is better—for now.Mov Disord. 2015 Apr;30(4):443-5. doi: 10.1002/mds.26169. Epub 2015 Mar 11. Mov Disord. 2015. PMID: 25757898 No abstract available.
-
Reply to letter: Suicide in Parkinson's disease patients treated with levodopa-carbidopa Intestinal Gel.Mov Disord. 2015 Sep;30(10):1435-6. doi: 10.1002/mds.26316. Epub 2015 Jul 16. Mov Disord. 2015. PMID: 26179940 Free PMC article. No abstract available.
-
Suicide in Parkinson's Disease Patients Treated With Levodopa-Carbidopa Intestinal Gel.Mov Disord. 2015 Sep;30(10):1434-5. doi: 10.1002/mds.26315. Epub 2015 Jul 17. Mov Disord. 2015. PMID: 26184074 No abstract available.
References
-
- Hauser RA. Levodopa: past, present, and future. Eur Neurol. 2009;62:1–8. - PubMed
-
- Hornykiewicz O. A brief history of levodopa. J Neurol. 2010;257(Suppl 2):S249–S252. - PubMed
-
- Antonini A, Chaudhuri KR, Martinez-Martin P, Odin P. Oral and infusion levodopa-based strategies for managing motor complications in patients with Parkinson's disease. CNS Drugs. 2010;24:119–129. - PubMed
-
- Olanow CW, Obeso JA, Stocchi F. Continuous dopamine-receptor treatment of Parkinson's disease: scientific rationale and clinical implications. Lancet Neurol. 2006;5:677–687. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

