Neuroendocrine-autonomic integration in the paraventricular nucleus: novel roles for dendritically released neuropeptides
- PMID: 25546497
- PMCID: PMC4447596
- DOI: 10.1111/jne.12252
Neuroendocrine-autonomic integration in the paraventricular nucleus: novel roles for dendritically released neuropeptides
Abstract
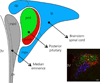
Communication between pairs of neurones in the central nervous system typically involves classical 'hard-wired' synaptic transmission, characterised by high temporal and spatial precision. Over the last two decades, however, knowledge regarding the repertoire of communication modalities used in the brain has notably expanded to include less conventional forms, characterised by a diffuse and less temporally precise transfer of information. These forms are best suited to mediate communication among entire neuronal populations, now recognised to be a fundamental process in the brain for the generation of complex behaviours. In response to an osmotic stressor, the hypothalamic paraventricular nucleus (PVN) generates a multimodal homeostatic response that involves orchestrated neuroendocrine (i.e. systemic release of vasopressin) and autonomic (i.e. sympathetic outflow to the kidneys) components. The precise mechanisms that underlie interpopulation cross-talk between these two distinct neuronal populations, however, remain largely unknown. The present review summarises and discusses a series of recent studies that have identified the dendritic release of neuropeptides as a novel interpopulation signalling modality in the PVN. A current working model is described in which it is proposed that the activity-dependent dendritic release of vasopressin from neurosecretory neurones in the PVN acts in a diffusible manner to increase the activity of distant presympathetic neurones, resulting in an integrated sympathoexcitatory population response, particularly within the context of a hyperosmotic challenge. The cellular mechanism underlying this novel form of intercellular communication, as well as its physiological and pathophysiological implications, is discussed.
Keywords: hypothalamus; neuroendocrine; osmotic; sympathetic; vasopressin.
© 2014 British Society for Neuroendocrinology.
Figures



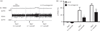

References
-
- Galarreta M, Hestrin S. Spike transmission and synchrony detection in networks of GABAergic interneurons. Science. 2001;292:2295–2299. - PubMed
-
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. - PubMed
-
- Lisman JE, Raghavachari S, Tsien RW. The sequence of events that underlie quantal transmission at central glutamatergic synapses. Nat Rev Neurosci. 2007;8:597–609. - PubMed
-
- Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. - PubMed
-
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

