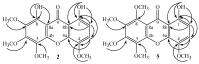Xanthones from the roots of Moutabea guianensis Aubl
- PMID: 25546625
- PMCID: PMC6272447
- DOI: 10.3390/molecules20010127
Xanthones from the roots of Moutabea guianensis Aubl
Abstract
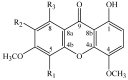
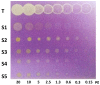
The phytochemical investigation of Moutabea guianensis roots led to the isolation of five polyoxygenated xanthones, including two new ones named moutabeone B (1,8-dihydroxy-4,5,6,7-tetramethoxyxanthone) and moutabeone C (1-hydroxy-4,5,6,7,8-pentamethoxyxanthone), along with the three known xanthones, 1,8-dihydroxy-4,6-dimethoxyxanthone, 1,8-dihydroxy-4,5,6-trimethoxyxanthone and augustin A (1,8-dihydroxy-4,6,7-trimethoxyxanthone). Structural characterization of all compounds was established on the basis of spectroscopic methods, mainly 1D and 2D nuclear magnetic resonance (NMR) and comparison with literature data. The antioxidant activity of compounds was tested through a thin layer chromatography (TLC) bioautography assay using 1,1-diphenyl-2-picryl-hydrazyl radical (DPPH·) as detection reagent. All tested compounds were more active (DL < 0.13-0.03 µg) than Trolox (DL < 0.15 µg), used as reference standard.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Cardona M.L., Fernándes I., Pedro J.R., Serrano A. Xanthones from Hypericum reflexum. Phytochemistry. 1990;29:3003–3006. doi: 10.1016/0031-9422(90)87123-C. - DOI
-
- Rocha J.L.C., Pastore J.F.B., Brandão H.N., Azevedo A., David J.P., Santos E.O., David J.M. Quantificação de salicilato de metila em quatro gêneros de Polygalaceae, por CLAE-DAD. Quim. Nova. 2012;35:2263–2266. doi: 10.1590/S0100-40422012001100033. - DOI
-
- Peres V., Nagem T.J. Naturally occurring pentaoxygenated, hexaoxyganated and dimeric xanthones: A literature survey. Quim. Nova. 1997;20:388–397. doi: 10.1590/S0100-40421997000400009. - DOI
-
- Dibwe D.F., Awale S., Kadota S., Morita H., Tezuka Y. Muchimangins E and F: Novel diphenylmethyl-substituted xanthones from Securidaca longepedunculata. Tetrahedron Lett. 2014;55:1916–1919. doi: 10.1016/j.tetlet.2014.01.149. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources