Poly-ADP ribosylation of PTEN by tankyrases promotes PTEN degradation and tumor growth
- PMID: 25547115
- PMCID: PMC4298135
- DOI: 10.1101/gad.251785.114
Poly-ADP ribosylation of PTEN by tankyrases promotes PTEN degradation and tumor growth
Abstract
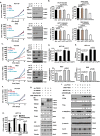
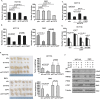
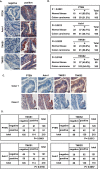
PTEN [phosphatidylinositol (3,4,5)-trisphosphate phosphatase and tensin homolog deleted from chromosome 10], a phosphatase and critical tumor suppressor, is regulated by numerous post-translational modifications, including phosphorylation, ubiquitination, acetylation, and SUMOylation, which affect PTEN localization and protein stability. Here we report ADP-ribosylation as a new post-translational modification of PTEN. We identified PTEN as a novel substrate of tankyrases, which are members of the poly(ADP-ribose) polymerases (PARPs). We showed that tankyrases interact with and ribosylate PTEN, which promotes the recognition of PTEN by a PAR-binding E3 ubiquitin ligase, RNF146, leading to PTEN ubiquitination and degradation. Double knockdown of tankyrase1/2 stabilized PTEN, resulting in the subsequent down-regulation of AKT phosphorylation and thus suppressed cell proliferation and glycolysis in vitro and tumor growth in vivo. Furthermore, tankyrases were up-regulated and negatively correlated with PTEN expression in human colon carcinomas. Together, our study revealed a new regulation of PTEN and highlighted a role for tankyrases in the PTEN-AKT pathway that can be explored further for cancer treatment.
Keywords: PARsylation; PTEN; RNF146; tankyrase; ubiquitination.
© 2015 Li et al.; Published by Cold Spring Harbor Laboratory Press.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
