Phase II, multicenter, open-label, randomized study of YM155 plus docetaxel as first-line treatment in patients with HER2-negative metastatic breast cancer
- PMID: 25547219
- PMCID: PMC4298663
- DOI: 10.1007/s10549-014-3238-6
Phase II, multicenter, open-label, randomized study of YM155 plus docetaxel as first-line treatment in patients with HER2-negative metastatic breast cancer
Abstract
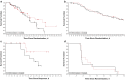
The objective of this study was to assess the efficacy and tolerability of YM155, a survivin suppressor, in combination with docetaxel, compared with docetaxel alone in patients with HER2-negative metastatic breast cancer. This phase II, multicenter, open-label, 2-arm study randomized patients (≥18 years) with histologically or cytologically confirmed stage IV HER2-negative metastatic breast cancer and ≥1 measurable lesion, to receive docetaxel alone or docetaxel plus YM155. The primary endpoint was progression-free survival (PFS). Secondary endpoints included objective response rate (ORR), overall survival (OS), duration of response (DOR), clinical benefit rate (CBR), time to response (TTR), biomarker assessment, and analysis of circulating tumor cells. Patients were women diagnosed with HER2-negative breast cancer; most had received prior drug therapies. The median PFS was 8.4 months with YM155 plus docetaxel (n = 50) and 10.5 months with docetaxel alone (n = 51; HR 1.53; 95 % CI 0.83, 2.83; P = 0.176). No statistically significant differences were observed for secondary endpoints, although slightly greater OS (630 vs 601 days; P = 0.768), CBR (84.3 vs 82.0 %; P = 0.855), DOR, and TTR were observed with docetaxel alone compared with YM155 plus docetaxel, whereas ORR was similar (25.5 vs 26.0). The most common TEAEs observed with YM155 plus docetaxel compared with docetaxel alone were neutropenia (83.3 vs 84.3 %), alopecia (62.5 vs 52.9 %), fatigue (50 vs 41.2 %), and nausea (37.5 vs 41.2 %). Although YM155 is a novel drug that suppresses survivin, YM155 plus docetaxel exhibited no statistically significant differences in endpoints compared with docetaxel alone. The combination regimen was well tolerated.
Figures
References
-
- Global Cancer Facts & Figures. (2011) American Cancer Society. Available via http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/docume.... Accessed 22 May 2014
-
- About Breast Cancer: Stages 0 and 1 (2012) National Breast Cancer Foundation, Inc. Available via http://www.nationalbreastcancer.org/breast-cancer-stage-0-and-stage-1. Accessed 15 May 2014
-
- Most common statistics cited for MBC (2014) Metastatic Breast Cancer Network. Available via http://mbcn.org/education/category/most-commonly-used-statistics-for-mbc. Accessed 15 May 2014
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous