Rapid testing using the Verigene Gram-negative blood culture nucleic acid test in combination with antimicrobial stewardship intervention against Gram-negative bacteremia
- PMID: 25547353
- PMCID: PMC4325795
- DOI: 10.1128/AAC.04259-14
Rapid testing using the Verigene Gram-negative blood culture nucleic acid test in combination with antimicrobial stewardship intervention against Gram-negative bacteremia
Abstract
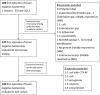
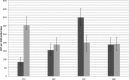
Rapid identification of microorganisms and antimicrobial resistance is paramount for targeted treatment in serious bloodstream infections (BSI). The Verigene Gram-negative blood culture nucleic acid test (BC-GN) is a multiplex, automated molecular diagnostic test for identification of eight Gram-negative (GN) organisms and resistance markers from blood culture with a turnaround time of approximately 2 h. Clinical isolates from adult patients at the University Maryland Medical Center with GN bacteremia from 1 January 2012 to 30 June 2012 were included in this study. Blood culture bottles were spiked with clinical isolates, allowed to incubate, and processed by BC-GN. A diagnostic evaluation was performed. In addition, a theoretical evaluation of time to effective and optimal antibiotic was performed, comparing actual antibiotic administration times from chart review ("control") to theoretical administration times based on BC-GN reporting and antimicrobial stewardship team (AST) review ("intervention"). For organisms detected by the assay, BC-GN correctly identified 95.6% (131/137), with a sensitivity of 97.1% (95% confidence interval [CI], 90.7 to 98.4%) and a specificity of 99.5% (95% CI, 98.8 to 99.8%). CTX-M and OXA resistance determinants were both detected. Allowing 12 h from Gram stain for antibiotic implementation, the intervention group had a significantly shorter duration to both effective (3.3 versus 7.0 h; P < 0.01) and optimal (23.5 versus 41.8 h; P < 0.01) antibiotic therapy. BC-GN with AST intervention can potentially decrease time to both effective and optimal antibiotic therapy in GN BSI.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, Oh MD, Choe KW. 2005. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother 49:760–766. doi:10.1128/AAC.49.2.760-766.2005. - DOI - PMC - PubMed
-
- Nanosphere, Inc. February 2014. Verigene Gram-negative blood culture nucleic acid test (BC-GN) package insert 027-00039-01, Rev B. Nanosphere Inc, Northbrook, IL.
-
- Mancini N, Infurnari L, Ghidoli N, Valzano G, Clementi N, Burioni R, Clementi M. 2014. Potential impact of a microarray-based nucleic acid assay for rapid detection of Gram-negative bacteria and resistance markers in positive blood cultures. J Clin Microbiol 52:1242–1245. doi:10.1128/JCM.00142-14. - DOI - PMC - PubMed
-
- Tojo M, Fujita T, Ainoda Y, Nagamatsu M, Hayakawa K, Mezaki K, Sakurai A, Masui Y, Yazaki H, Takahashi H, Miyoshi-Akiyama T, Totsuka K, Kirikae T, Ohmagari N. 2014. Evaluation of an automated rapid diagnostic assay for detection of gram-negative bacteria and their drug-resistance genes in positive blood cultures. PLoS One 9:e9406. doi:10.1371/journal.pone.0094064. - DOI - PMC - PubMed
-
- Sullivan KV, Deburger B, Roundtree SS, Ventrola CA, Blecker-Shelly DL, Mortensen JE. 2014. Pediatric multicenter evaluation of the Verigene Gram-negative blood culture test for rapid detection of inpatient bacteremia involving Gram-negative organisms, extended-spectrum beta-lactamases, and carbapenemases. J Clin Microbiol 52:2416–2421. doi:10.1128/JCM.00737-14. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

