A cognitive-emotional biomarker for predicting remission with antidepressant medications: a report from the iSPOT-D trial
- PMID: 25547711
- PMCID: PMC4397406
- DOI: 10.1038/npp.2014.333
A cognitive-emotional biomarker for predicting remission with antidepressant medications: a report from the iSPOT-D trial
Abstract
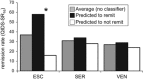
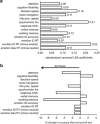
Depression involves impairments in a range of cognitive and emotional capacities. It is unknown whether these functions can inform medication choice when considered as a composite predictive biomarker. We tested whether behavioral tests, grounded in the neurobiology of cognitive and emotional functions, predict outcome with common antidepressants. Medication-free outpatients with nonpsychotic major depressive disorder (N=1008; 665 completers) were assessed before treatment using 13 computerized tests of psychomotor, executive, memory-attention, processing speed, inhibitory, and emotional functions. Matched healthy controls (N=336) provided a normative reference sample for test performance. Depressed participants were then randomized to escitalopram, sertraline, or venlafaxine-extended release, and were assessed using the 16-item Quick Inventory of Depressive Symptomatology (QIDS-SR16) and the 17-item Hamilton Rating Scale for Depression. Given the heterogeneity of depression, analyses were furthermore stratified by pretreatment performance. We then used pattern classification with cross-validation to determine individual patient-level composite predictive biomarkers of antidepressant outcome based on test performance. A subgroup of depressed participants (approximately one-quarter of patients) were found to be impaired across most cognitive tests relative to the healthy norm, from which they could be discriminated with 91% accuracy. These patients with generally impaired cognitive task performance had poorer treatment outcomes. For this impaired subgroup, task performance furthermore predicted remission on the QIDS-SR16 at 72% accuracy specifically following treatment with escitalopram but not the other medications. Therefore, tests of cognitive and emotional functions can form a clinically meaningful composite biomarker that may help drive general treatment outcome prediction for optimal treatment selection in depression, particularly for escitalopram.
Figures
References
-
- American Psychiatric Association 1994Diagnostic and Statistical Manual of Mental Disorders4th ednAmerican Psychiatric Association: Washington, DC
-
- Dunkin JJ, Leuchter AF, Cook IA, Kasl-Godley JE, Abrams M, Rosenberg-Thompson S. Executive dysfunction predicts nonresponse to fluoxetine in major depression. J Affect Disord. 2000;60:13–23. - PubMed
-
- Dunn OJ. Multiple comparisons among means. J Am Stat Assoc. 1961;56:52–64.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

