Loss of feedback inhibition via D2 autoreceptors enhances acquisition of cocaine taking and reactivity to drug-paired cues
- PMID: 25547712
- PMCID: PMC4397408
- DOI: 10.1038/npp.2014.336
Loss of feedback inhibition via D2 autoreceptors enhances acquisition of cocaine taking and reactivity to drug-paired cues
Abstract
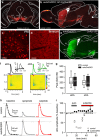
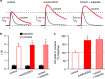
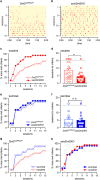
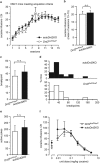
A prominent aspect of drug addiction is the ability of drug-associated cues to elicit craving and facilitate relapse. Understanding the factors that regulate cue reactivity will be vital for improving treatment of addictive disorders. Low availability of dopamine (DA) D2 receptors (D2Rs) in the striatum is associated with high cocaine intake and compulsive use. However, the role of D2Rs of nonstriatal origin in cocaine seeking and taking behavior and cue reactivity is less understood and possibly underestimated. D2Rs expressed by midbrain DA neurons function as autoreceptors, exerting inhibitory feedback on DA synthesis and release. Here, we show that selective loss of D2 autoreceptors impairs the feedback inhibition of DA release and amplifies the effect of cocaine on DA transmission in the nucleus accumbens (NAc) in vitro. Mice lacking D2 autoreceptors acquire a cued-operant self-administration task for cocaine faster than littermate control mice but acquire similarly for a natural reward. Furthermore, although mice lacking D2 autoreceptors were able to extinguish self-administration behavior in the absence of cocaine and paired cues, they exhibited perseverative responding when cocaine-paired cues were present. This enhanced cue reactivity was selective for cocaine and was not seen during extinction of sucrose self-administration. We conclude that low levels of D2 autoreceptors enhance the salience of cocaine-paired cues and can contribute to the vulnerability for cocaine use and relapse.
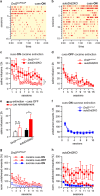
Figures
References
-
- Alcantara AA, Chen V, Herring BE, Mendenhall JM, Berlanga ML. Localization of dopamine D2 receptors on cholinergic interneurons of the dorsal striatum and nucleus accumbens of the rat. Brain Res. 2003;986:22–29. - PubMed
-
- Bertorelli R, Consolo S. D1 and D2 dopaminergic regulation of acetylcholine release from striata of freely moving rats. J Neurochem. 1990;54:2145–2148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

