Endocannabinoid signaling within the basolateral amygdala integrates multiple stress hormone effects on memory consolidation
- PMID: 25547713
- PMCID: PMC4397407
- DOI: 10.1038/npp.2014.334
Endocannabinoid signaling within the basolateral amygdala integrates multiple stress hormone effects on memory consolidation
Abstract
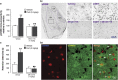
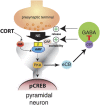
Glucocorticoid hormones are known to act synergistically with other stress-activated neuromodulatory systems, such as norepinephrine and corticotropin-releasing factor (CRF), within the basolateral complex of the amygdala (BLA) to induce optimal strengthening of the consolidation of long-term memory of emotionally arousing experiences. However, as the onset of these glucocorticoid actions appear often too rapid to be explained by genomic regulation, the neurobiological mechanism of how glucocorticoids could modify the memory-enhancing properties of norepinephrine and CRF remained elusive. Here, we show that the endocannabinoid system, a rapidly activated retrograde messenger system, is a primary route mediating the actions of glucocorticoids, via a glucocorticoid receptor on the cell surface, on BLA neural plasticity and memory consolidation. Furthermore, glucocorticoids recruit downstream endocannabinoid activity within the BLA to interact with both the norepinephrine and CRF systems in enhancing memory consolidation. These findings have important implications for understanding the fine-tuned crosstalk between multiple stress hormone systems in the coordination of (mal)adaptive stress and emotional arousal effects on neural plasticity and memory consolidation.
Figures

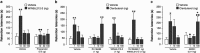

References
-
- Atsak P, Roozendaal B, Campolongo P. Role of the endocannabinoid system in regulating glucocorticoid effects on memory for emotional experiences. Neuroscience. 2012;204:104–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

