Efficacy and safety of autologous platelet rich plasma for the treatment of vascular ulcers in primary care: Phase III study
- PMID: 25547983
- PMCID: PMC4311495
- DOI: 10.1186/s12875-014-0211-8
Efficacy and safety of autologous platelet rich plasma for the treatment of vascular ulcers in primary care: Phase III study
Abstract
Background: Vascular ulcers are commonly seen in daily practice at all levels of care and have great impact at personal, professional and social levels with a high cost in terms of human and material resources. Given that the application of autologous platelet rich plasma has been shown to decrease healing times in various different studies in the hospital setting, we considered that it would be interesting to assess the efficacy and feasibility of this treatment in primary care. The objectives of this study are to assess the potential efficacy and safety of autologous platelet rich plasma for the treatment of venous ulcers compared to the conventional treatment (moist wound care) in primary care patients with chronic venous insufficiency (C, clinical class, E, aetiology, A, anatomy and P, pathophysiology classification C6).
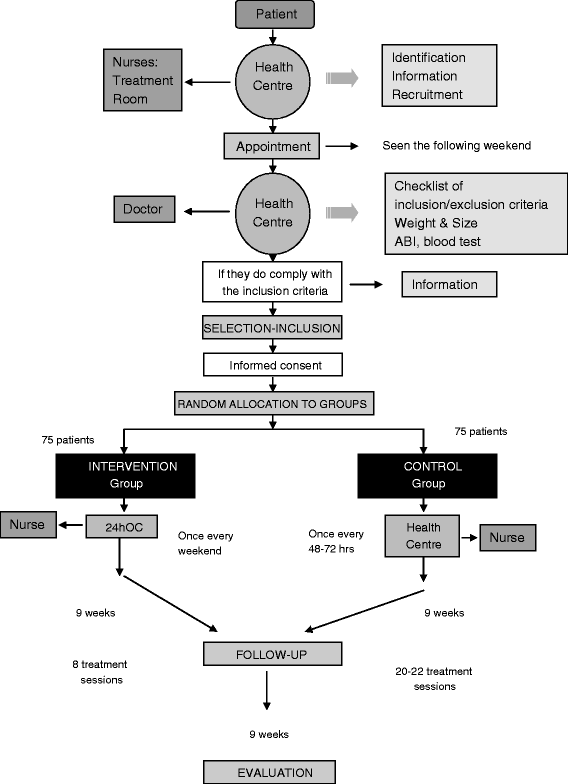
Design: We will conduct a phase III, open-label, parallel-group, multicentre, randomized study. The subjects will be 150 patients aged between 40 and 100 years of age with an at least 2-month history of a vascular venous ulcer assigned to ten primary care centres. For the treatment with autologous platelet rich plasma, all the following tasks will be performed in the primary care setting: blood collection, centrifugation, separation of platelet rich plasma, activation of coagulation adding calcium chloride and application of the PRP topically after gelification. The control group will receive standard moist wound care. The outcome variables to be measured at baseline, and at weeks 5 and 9 later include: reduction in the ulcer area, Chronic Venous Insufficiency Quality of Life Questionnaire score, and percentage of patients who require wound care only once a week.
Discussion: The results of this study will be useful to improve the protocol for using platelet rich plasma in chronic vascular ulcers and to favour wider use of this treatment in primary care.
Trial registration: Current Controlled Trials NCT02213952.
References
-
- Mast BA. The skin. In: Cohen IK, Diegelmann RF, Lindblad WJ, editors. Wound Healing, Biochemical and Clinical Aspects. Philadelphia, PA: W.B. Saunders; 1992.
-
- Lawrence WT. Clinical management of nonhealing wounds. In: Cohen IK, Diegelmann RF, Lindblad WJ, editors. Wound Healing: Biochemical and Clinical Aspects. Philadelphia, PA: W.B. Saunders; 1992. pp. 541–61.
-
- Gesto-Castromil R, Grupo DETECT-IVC, García JJ. Encuesta epidemiológica realizada en España sobre la prevalencia asistencial de la insuficiencia venosa crónica en atención primaria. Estudio DETECT-IVC. Angiología. 2001;53:249–260.
-
- Brand FN, Dannenberg AL, Abbort RD, Kannel WB. The epidemiology of varicose veins: The Framingham study. Am J Prev Med. 1988;4:96–101. - PubMed
-
- Stvrtinova V, Kolesar J, Wimmer G. Prevalence of varicose veins of the lower limbs in the women working at a department store. Int Angiol. 1991;10:2–5. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials