Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter
- PMID: 25548026
- PMCID: PMC4297708
- DOI: 10.1016/j.drugalcdep.2014.12.005
Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter
Abstract
Background: Treatment of stimulant-use disorders remains a formidable challenge, and the dopamine transporter (DAT) remains a potential target for antagonist or agonist-like substitution therapies.
Methods: This review focuses on DAT ligands, such as benztropine, GBR 12909, modafinil, and DAT substrates derived from phenethylamine or cathinone that have atypical DAT-inhibitor effects, either in vitro or in vivo. The compounds are described from a molecular mechanistic, behavioral, and medicinal-chemical perspective.
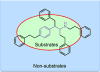
Results: Possible mechanisms for atypicality at the molecular level can be deduced from the conformational cycle for substrate translocation. For each conformation, a crystal structure of a bacterial homolog is available, with a possible role of cholesterol, which is also present in the crystal of Drosophila DAT. Although there is a direct relationship between behavioral potencies of most DAT inhibitors and their DAT affinities, a number of compounds bind to the DAT and inhibit dopamine uptake but do not share cocaine-like effects. Such atypical behavior, depending on the compound, may be related to slow DAT association, combined sigma-receptor actions, or bias for cytosol-facing DAT. Some structures are sterically small enough to serve as DAT substrates but large enough to also inhibit transport. Such compounds may display partial DA releasing effects, and may be combined with release or uptake inhibition at other monoamine transporters.
Conclusions: Mechanisms of atypical DAT inhibitors may serve as targets for the development of treatments for stimulant abuse. These mechanisms are novel and their further exploration may produce compounds with unique therapeutic potential as treatments for stimulant abuse.
Keywords: Atypical effects; DA releasers; DAT inhibitors; Dopamine transporter; Molecular mechanisms; Stimulant abuse treatment compounds.
Copyright © 2014 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Figures
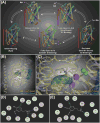
 +) or aromatic π-stacking (
+) or aromatic π-stacking (

 ).
).

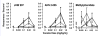
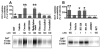

References
-
- Adkins EM, Samuvel DJ, Fog JU, Eriksen J, Jayanthi LD, Vaegter CB, Ramamoorthy S, Gether U. Membrane mobility and microdomain association of the dopamine transporter studied with fluorescence correlation spectroscopy and fluorescence recovery after photobleaching. Biochemistry. 2007;46:10484–10497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

