Field and experimental evidence of Vibrio parahaemolyticus as the causative agent of acute hepatopancreatic necrosis disease of cultured shrimp (Litopenaeus vannamei) in Northwestern Mexico
- PMID: 25548045
- PMCID: PMC4325143
- DOI: 10.1128/AEM.03610-14
Field and experimental evidence of Vibrio parahaemolyticus as the causative agent of acute hepatopancreatic necrosis disease of cultured shrimp (Litopenaeus vannamei) in Northwestern Mexico
Abstract
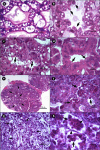
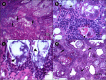
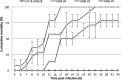
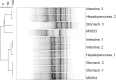
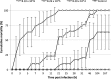
Moribund shrimp affected by acute hepatopancreatic necrosis disease (AHPND) from farms in northwestern Mexico were sampled for bacteriological and histological analysis. Bacterial isolates were molecularly identified as Vibrio parahaemolyticus by the presence of the tlh gene. The tdh-negative, trh-negative, and tlh-positive V. parahaemolyticus strains were further characterized by repetitive extragenic palindromic element-PCR (rep-PCR), and primers AP1, AP2, AP3, and AP and an ems2 IQ2000 detection kit (GeneReach, Taiwan) were used in the diagnostic tests for AHPND. The V. parahaemolyticus strains were used in immersion challenges with shrimp, and farmed and challenged shrimp presented the same clinical and pathological symptoms: lethargy, empty gut, pale and aqueous hepatopancreas, and expanded chromatophores. Using histological analysis and bacterial density count, three stages of AHNPD (initial, acute, and terminal) were identified in the affected shrimp. The pathognomonic lesions indicating severe desquamation of tubular epithelial cells of the hepatopancreas were observed in both challenged and pond-infected shrimp. The results showed that different V. parahaemolyticus strains have different virulences; some of the less virulent strains do not induce 100% mortality, and mortality rates also rise more slowly than they do for the more virulent strains. The virulence of V. parahaemolyticus strains was dose dependent, where the threshold infective density was 10(4) CFU ml(-1); below that density, no mortality was observed. The AP3 primer set had the best sensitivity and specificity. Field and experimental results showed that the V. parahaemolyticus strain that causes AHPND acts as a primary pathogen for shrimp in Mexico compared with the V. parahaemolyticus strains reported to date.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Lightner DV. 1999. The Penaeid Shrimp Viruses TSV, IHHNV, WSSV, and YHV: current status in the Americas, available diagnostic methods, and management strategies. J Appl Aquacult 9:27–52. doi: 10.1300/J028v09n02_03. - DOI
-
- Del Rio-Rodriguez RE, Soto-Rodriguez S, Lara-Flores M, Cu-Escamilla AD, Gomez-Solano MI. 2006. A necrotizing hepatopancreatitis (NHP) outbreak in a shrimp farm in Campeche, Mexico: a first case report. Aquaculture 255:606–209. doi: 10.1016/j.aquaculture.2005.12.014. - DOI
-
- Nunan L, Pantoja C, Gomez-Jimenez S, Lightner D. 2013. “Candidatus hepatobacter penaei,” an intracellular pathogenic enteric bacterium in the hepatopancreas of the marine shrimp Penaeus vannamei (Crustacea: Decapoda). Appl Environ Microbiol 79:1407–1409. doi: 10.1128/AEM.02425-12. - DOI - PMC - PubMed
-
- Soto-Rodriguez SA, Gomez Gil B, Roque A., Lozano R. 2010. Density of vibrios in hemolymph and hepatopancreas of diseased Pacific white shrimp, Litopenaeus vannamei, from northwestern Mexico. J World Aquacult Soc 41:76–83. doi: 10.1111/j.1749-7345.2009.00335.x. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

