Bacterial growth laws reflect the evolutionary importance of energy efficiency
- PMID: 25548180
- PMCID: PMC4299221
- DOI: 10.1073/pnas.1421138111
Bacterial growth laws reflect the evolutionary importance of energy efficiency
Abstract
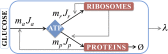
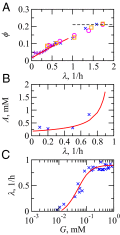
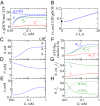
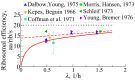
We are interested in the balance of energy and protein synthesis in bacterial growth. How has evolution optimized this balance? We describe an analytical model that leverages extensive literature data on growth laws to infer the underlying fitness landscape and to draw inferences about what evolution has optimized in Escherichia coli. Is E. coli optimized for growth speed, energy efficiency, or some other property? Experimental data show that at its replication speed limit, E. coli produces about four mass equivalents of nonribosomal proteins for every mass equivalent of ribosomes. This ratio can be explained if the cell's fitness function is the the energy efficiency of cells under fast growth conditions, indicating a tradeoff between the high energy costs of ribosomes under fast growth and the high energy costs of turning over nonribosomal proteins under slow growth. This model gives insight into some of the complex nonlinear relationships between energy utilization and ribosomal and nonribosomal production as a function of cell growth conditions.
Keywords: bacterial metabolism; energy efficiency; fitness landscape; growth laws; yield.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Monod J. The growth of bacterial cultures. Annu Rev Microbiol. 1949;3:371–394.
-
- Hinshelwood CN. On the chemical kinetics of autosynthetic systems. J Chem Soc. 1952:745–755.
-
- Ehrenberg M, Kurland CG. Costs of accuracy determined by a maximal growth rate constraint. Q Rev Biophys. 1984;17(1):45–82. - PubMed
-
- Koch AL. Why can’t a cell grow infinitely fast? Can J Microbiol. 1988;34(4):421–426. - PubMed
-
- Cox RA. Quantitative relationships for specific growth rates and macromolecular compositions of Mycobacterium tuberculosis, Streptomyces coelicolor A3(2) and Escherichia coli B/r: An integrative theoretical approach. Microbiology. 2004;150(Pt 5):1413–1426. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

