Induction and activation of latent transforming growth factor-β1 are carried out by two distinct domains of pregnancy-specific glycoprotein 1 (PSG1)
- PMID: 25548275
- PMCID: PMC4326847
- DOI: 10.1074/jbc.M114.597518
Induction and activation of latent transforming growth factor-β1 are carried out by two distinct domains of pregnancy-specific glycoprotein 1 (PSG1)
Abstract
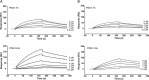
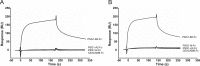
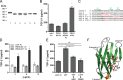
Pregnancy-specific glycoproteins (PSGs) are a family of Ig-like proteins secreted by specialized placental cells. The PSG1 structure is composed of a single Ig variable region-like N-terminal domain and three Ig constant region-like domains termed A1, A2, and B2. Members of the human and murine PSG family have been shown to induce anti-inflammatory cytokines from monocytes and macrophages and to stimulate angiogenesis. We recently showed that recombinant forms of PSG1 (PSG1-Fc and PSG1-His) and PSG1 purified from the serum of pregnant women are associated with the immunoregulatory cytokine TGF-β1 and activated latent TGF-β1. Here, we sought to examine the requirement of specific PSG1 domains in the activation of latent TGF-β1. Plasmon surface resonance studies showed that PSG1 directly bound to the small latent complex and to the latency-associated peptide of TGF-β1 and that this binding was mediated through the B2 domain. Furthermore, the B2 domain alone was sufficient for activating the small latent complex. In separate experiments, we found that the PSG1-mediated induction of TGF-β1 secretion in macrophages was dependent on the N-terminal domain. Mutagenesis analysis revealed that four amino acids (LYHY) of the CC' loop of the N-terminal domain were required for induction of latent TGF-β1 secretion. Together, our results show that two distinct domains of PSG1 are involved in the regulation of TGF-β1 and provide a mechanistic framework for how PSGs modulate the immunoregulatory environment at the maternal-fetal interface for successful pregnancy outcome.
Keywords: Heparan Sulfate; Latency-associated Peptide; Latent TGF-β1; Macrophage; Placenta; Pregnancy; Surface Plasmon Resonance (SPR).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Teglund S., Olsen A., Khan W. N., Frängsmyr L., Hammarström S. (1994) The pregnancy-specific glycoprotein (PSG) gene cluster on human chromosome 19: fine structure of the 11 PSG genes and identification of 6 new genes forming a third subgroup within the carcinoembryonic antigen (CEA) family. Genomics 23, 669–684 - PubMed
-
- Snyder S. K., Wessner D. H., Wessells J. L., Waterhouse R. M., Wahl L. M., Zimmermann W., Dveksler G. S. (2001) Pregnancy-specific glycoproteins function as immunomodulators by inducing secretion of IL-10, IL-6 and TGF-β1 by human monocytes. Am. J. Reprod. Immunol. 45, 205–216 - PubMed
-
- Martínez F. F., Knubel C. P., Sánchez M. C., Cervi L., Motrán C. C. (2012) Pregnancy-specific glycoprotein 1a activates dendritic cells to provide signals for Th17-, Th2-, and Treg-cell polarization. Eur. J. Immunol. 42, 1573–1584 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

