Text message reminders for second dose of influenza vaccine: a randomized controlled trial
- PMID: 25548329
- PMCID: PMC4279072
- DOI: 10.1542/peds.2014-2475
Text message reminders for second dose of influenza vaccine: a randomized controlled trial
Abstract
Objective: To determine whether provision of vaccine-health-literacy-promoting information in text message vaccine reminders improves receipt and timeliness of the second dose of influenza vaccine within a season for children in need of 2 doses.
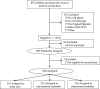
Methods: During the 2012-2013 season, families of eligible 6-month through 8-year-old children were recruited at the time of their first influenza vaccination from 3 community clinics in New York City. Children (n = 660) were randomly assigned to "educational" text message, "conventional" text message, and "written reminder-only" arms. At enrollment, all arms received a written reminder with next dose due date. Conventional messages included second dose due date and clinic walk-in hours. Educational messages added information regarding the need for a timely second dose. Receipt of second dose by April 30 was assessed by using χ(2) tests. Timeliness was assessed by receipt of second dose by 2 weeks after due date (day 42) using χ(2) and over time using a Kaplan-Meier analysis.
Results: Most families were Latino and publicly insured with no significant between-arm differences between groups. Children in the educational arm were more likely to receive a second dose by April 30 (72.7%) versus conventional (66.7%) versus written reminder-only arm (57.1%; P = .003). They also had more timely receipt by day 42 (P < .001) and over time (P < .001).
Conclusions: In this low-income, urban, minority population, embedding health literacy information improved the effectiveness of text message reminders in promoting timely delivery of a second dose of influenza vaccine, compared with conventional text messages and written reminder only.
Trial registration: ClinicalTrials.gov NCT01662583.
Keywords: immunizations; influenza; mobile health; reminder-recall; text message.
Copyright © 2015 by the American Academy of Pediatrics.
Figures

References
-
- Moore DL, Vaudry W, Scheifele DW, et al. . Surveillance for influenza admissions among children hospitalized in Canadian immunization monitoring program active centers, 2003-2004. Pediatrics. 2006;118(3). Available at: www.pediatrics.org/cgi/content/full/118/3/e610 - PubMed
-
- Thompson WW, Shay DK, Weintraub E, et al. . Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333–1340 - PubMed
-
- Louie JK, Schechter R, Honarmand S, et al. . Severe pediatric influenza in California, 2003-2005: implications for immunization recommendations. Pediatrics. 2006;117(4). Available at: www.pediatrics.org/cgi/content/full/117/4/e610 - PubMed
-
- Neuzil KM, Zhu Y, Griffin MR, et al. . Burden of interpandemic influenza in children younger than 5 years: a 25-year prospective study. J Infect Dis. 2002;185(2):147–152 - PubMed
-
- Fiore AE, Uyeki TM, Broder K, et al. Centers for Disease Control and Prevention (CDC) . Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1–62 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

