Pathogenesis of alcoholic liver disease: role of oxidative metabolism
- PMID: 25548474
- PMCID: PMC4273126
- DOI: 10.3748/wjg.v20.i47.17756
Pathogenesis of alcoholic liver disease: role of oxidative metabolism
Abstract
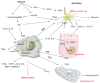
Alcohol consumption is a predominant etiological factor in the pathogenesis of chronic liver diseases, resulting in fatty liver, alcoholic hepatitis, fibrosis/cirrhosis, and hepatocellular carcinoma (HCC). Although the pathogenesis of alcoholic liver disease (ALD) involves complex and still unclear biological processes, the oxidative metabolites of ethanol such as acetaldehyde and reactive oxygen species (ROS) play a preeminent role in the clinical and pathological spectrum of ALD. Ethanol oxidative metabolism influences intracellular signaling pathways and deranges the transcriptional control of several genes, leading to fat accumulation, fibrogenesis and activation of innate and adaptive immunity. Acetaldehyde is known to be toxic to the liver and alters lipid homeostasis, decreasing peroxisome proliferator-activated receptors and increasing sterol regulatory element binding protein activity via an AMP-activated protein kinase (AMPK)-dependent mechanism. AMPK activation by ROS modulates autophagy, which has an important role in removing lipid droplets. Acetaldehyde and aldehydes generated from lipid peroxidation induce collagen synthesis by their ability to form protein adducts that activate transforming-growth-factor-β-dependent and independent profibrogenic pathways in activated hepatic stellate cells (HSCs). Furthermore, activation of innate and adaptive immunity in response to ethanol metabolism plays a key role in the development and progression of ALD. Acetaldehyde alters the intestinal barrier and promote lipopolysaccharide (LPS) translocation by disrupting tight and adherent junctions in human colonic mucosa. Acetaldehyde and LPS induce Kupffer cells to release ROS and proinflammatory cytokines and chemokines that contribute to neutrophils infiltration. In addition, alcohol consumption inhibits natural killer cells that are cytotoxic to HSCs and thus have an important antifibrotic function in the liver. Ethanol metabolism may also interfere with cell-mediated adaptive immunity by impairing proteasome function in macrophages and dendritic cells, and consequently alters allogenic antigen presentation. Finally, acetaldehyde and ROS have a role in alcohol-related carcinogenesis because they can form DNA adducts that are prone to mutagenesis, and they interfere with methylation, synthesis and repair of DNA, thereby increasing HCC susceptibility.
Keywords: Acetaldehyde; Alcohol metabolism; Alcoholic liver disease; CYP2E1; Hepatic stellate cells; Liver fibrosis; Protein adducts; Reactive oxygen species.
Figures


References
-
- Wilfred de Alwis NM, Day CP. Genetics of alcoholic liver disease and nonalcoholic fatty liver disease. Semin Liver Dis. 2007;27:44–54. - PubMed
-
- Kimura M, Miyakawa T, Matsushita S, So M, Higuchi S. Gender differences in the effects of ADH1B and ALDH2 polymorphisms on alcoholism. Alcohol Clin Exp Res. 2011;35:1923–1927. - PubMed
-
- Bosron WF, Ehrig T, Li TK. Genetic factors in alcohol metabolism and alcoholism. Semin Liver Dis. 1993;13:126–135. - PubMed
-
- Crabb DW. Ethanol oxidizing enzymes: roles in alcohol metabolism and alcoholic liver disease. Prog Liver Dis. 1995;13:151–172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

