Synthesis, characterization, and anticancer activity of new quinazoline derivatives against MCF-7 cells
- PMID: 25548779
- PMCID: PMC4274848
- DOI: 10.1155/2014/212096
Synthesis, characterization, and anticancer activity of new quinazoline derivatives against MCF-7 cells
Abstract
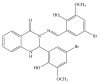
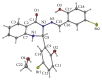
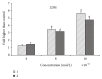
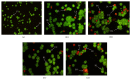
Two new synthesized and characterized quinazoline Schiff bases 1 and 2 were investigated for anticancer activity against MCF-7 human breast cancer cell line. Compounds 1 and 2 demonstrated a remarkable antiproliferative effect, with an IC50 value of 6.246×10(-6) mol/L and 5.910×10(-6) mol/L, respectively, after 72 hours of treatment. Most apoptosis morphological features in treated MCF-7 cells were observed by AO/PI staining. The results of cell cycle analysis indicate that compounds did not induce S and M phase arrest in cell after 24 hours of treatment. Furthermore, MCF-7 cells treated with 1 and 2 subjected to apoptosis death, as exhibited by perturbation of mitochondrial membrane potential and cytochrome c release as well as increase in ROS formation. We also found activation of caspases-3/7, -8, and -9 in compounds 1 and 2. Moreover, inhibition of NF-κB translocation in MCF-7 cells treated by compound 1 significantly exhibited the association of extrinsic apoptosis pathway. Acute toxicity results demonstrated the nontoxic nature of the compounds in mice. Our results showed significant activity towards MCF-7 cells via either intrinsic or extrinsic mitochondrial pathway and are potential candidate for further in vivo and clinical breast cancer studies.
Figures













References
-
- Saurav K., Garima M., Pradeep S., Jha K. K., Khosa R. L., Gupta S. K. Quinazoline-4-one: a highly important hetrocycle with diverse biological activities. Der Chemica Sinica. 2011;2(4):36–58.
-
- Selvam T. P., Kumar P. V., Vijayaraj P. Quinazoline marketed drugs—a review. Research in Pharmacy. 2011;1(1):1–21.
-
- A. Shetha, I. A. Wijdan Synthesis and characterization of new quinazoline-4(3H)-one Schiff bases. Journal of Chemical and Pharmaceutical Research. 2013;5(7):42–45.
-
- Vagdevi H. M., Lokesh M. R., Gowdarshivannanavar B. C. Synthesis and antioxidant activity of 3-substituted Schiff bases of quinazoline-2,4-diones. International Journal of ChemTech Research. 2012;4(4):1527–1533.
-
- Krishnan S. K., Ganguly S., Veerasamy R., Jan B. Synthesis, antiviral and cytotoxic investigation of 2-phenyl-3-substituted quinazolin-4(3H)-ones. European Review for Medical and Pharmacological Sciences. 2011;15(6):673–681. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

