Evaluation of hematocrit interference with MyStar extra and seven competitive devices
- PMID: 25549636
- PMCID: PMC4604595
- DOI: 10.1177/1932296814565790
Evaluation of hematocrit interference with MyStar extra and seven competitive devices
Abstract
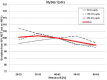
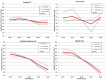
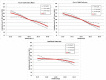
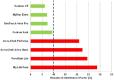
In previous studies, meters employing dynamic electrochemistry (DE), have been shown to correct for hematocrit (HCT) interference. This laboratory investigation assessed the HCT stability of MyStar Extra (Sanofi) in comparison to 7 competitive devices (Accu-Chek Aviva Nano & Accu-Chek Performa, Roche Diagnostics; Contour XT and Contour Link, Bayer; FreeStyle Freedom Lite, Abbott; MyLife Pura, Ypsomed; OneTouch Verio Pro, LifeScan). Venous heparinized blood was freshly drawn, immediately aliquoted, and manipulated to contain 3 different blood glucose concentrations (50-80 mg/dL, 150-180 mg/dL, and 350-400 mg/dL) and 5 different HCT levels (20-25%, 30-35%, 40-45%, 50-55%, and 60-65%). After careful oxygenation to normal blood oxygen pressure, each of the 15 different samples was measured 8 times with 2 devices and 2 strip lots of each meter (32 measurements/meter/sample). YSI Stat 2300 served as laboratory reference method. Next to determination of the mean absolute relative deviation (MARD), stability to HCT influence was assumed, when less than 10% difference occurred between the highest and lowest mean glucose deviations in relation to HCT over all tested glucose ranges (HIF: hematocrit interference factor). Four of the devices showed stable performance: Contour XT (MARD: 1.3%/HIF: 6.1%), MyStar Extra (4.7%/7.1%), OneTouch Verio Pro (4.5%/7.3%), and Contour Link (6.3%/9.3%). The 4 other meters were influenced by HCT (Accu-Chek Performa: 4.7%/20.9%, Accu-Chek Aviva Nano: 4.5%/22.4%, FreeStyle Freedom Lite: 4.8%/24.5%; MyLife Pura: 6.4%/28.7%). In this study, all meters showed a good accuracy, but only 50% of them, including MyStar Extra, were shown to reliably correct for potential hematocrit influence on the meter results.
Keywords: accuracy; blood glucose meter; dynamic electrochemistry; hematocrit interference.
© 2014 Diabetes Technology Society.
Conflict of interest statement
Figures
References
-
- Lyon ME, Lyon AW. Patient acuity exacerbates the discrepancy between whole blood and plasma methods through error in molality to molarity conversion: “Mind the gap!” Clin Biochem. 2011;44:412-417. - PubMed
-
- Volkova N, Arab L. Evidence-based systematic literature review of hemoglobin/hematocrit and all-cause mortality in dialysis patients. Am J Kidney Dis. 2006;47:24-36. - PubMed
-
- Thirup P. Haematocrit: within-subject and seasonal variation. Sports Med. 2003;33:231-243. - PubMed
-
- Takubo T, Tatsumi N. Reference values for hematologic laboratory tests and hematologic disorders in the aged. Rinsho Byori. 2000;48(3):207-216. - PubMed
-
- Macdougall IC, Ritz E. The Normal Haematocrit Trial in dialysis patients with cardiac disease: are we any the less confused about target haemoglobin? Nephrol Dial Transplant. 1998;13(12):3030-3033. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

