Phase separation behavior of mixed lipid systems at neutral and low pH: coarse-grained simulations with DMD/LIME
- PMID: 25549801
- PMCID: PMC4310635
- DOI: 10.1021/la504082x
Phase separation behavior of mixed lipid systems at neutral and low pH: coarse-grained simulations with DMD/LIME
Abstract
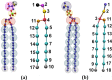
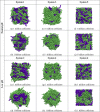
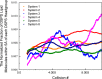
We extend LIME, an intermediate resolution, implicit solvent model for phospholipids previously used in discontinuous molecular dynamics simulations of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) bilayer formation at 325 K, to the description of the geometry and energetics of 1,2-distearoyl-sn-glycero-3-phospho-L-serine (DSPS) and 1,2-dihenarachidoyl-sn-glycero-3-phosphocholine (21PC) and mixtures thereof at both neutral and low pH at 310 K. A multiscale modeling approach is used to calculate the LIME parameters from atomistic simulation data on a mixed DPPC/DSPS system at different pH values. In the model, 17 coarse-grained sites represent DSPS and 18 coarse-grained sites represent 21PC. Each of these coarse-grained sites is classified as 1 of 9 types. LIME/DMD simulations of equimolar bilayers show the following: (1) 21PC/DSPS bilayers with and without surface area restrictions separate faster at low pH than at neutral pH, (2) 21PC/DSPS systems separate at approximately the same rate regardless of whether they are subjected to surface area restrictions, and (3) bilayers with a molar ratio of 9:1 (21PC:DSPS) phase separate to form heterogeneous domains faster at low pH than at neutral pH. Our results are consistent with experimental findings of Sofou and co-workers (Bandekar et al. Mol. Pharmaceutics, 2013, 10, 152-160; Karve et al. Biomaterials, 2010, 31, 4409-4416) that more doxorubicin is released from 21PC/DSPS liposomes at low pH than at neutral pH, presumably because greater phase separation is achieved at low pH than at neutral pH. These are the first molecular-level simulations of the phase separation in mixed lipid bilayers induced by a change in pH.
Figures

References
-
- Nikolelis D.; Hianik T.; Krull U. Biosensors Based on Thin Lipid Films and Liposomes. Electroanalysis 1999, 11, 7–15.
-
- Bally M.; Bailey K.; Sugihara K.; Grieshaber D.; Voros J.; Stadler B. Liposome and Lipid Bilayer Arrays towards Biosensing Applications. Small 2010, 6, 2481–2497. - PubMed
-
- Lingwood D.; Simons K. Lipid Rafts as a Membrane-Organizing Principle. Science 2010, 327, 46–50. - PubMed
-
- Mukherjee S.; Maxfield F. Role of Membrane Organization and Membrane Domains in Endocytic Lipid Trafficking. Traffic 2000, 1, 203–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

