Transmission of atherosclerosis susceptibility with gut microbial transplantation
- PMID: 25550161
- PMCID: PMC4342477
- DOI: 10.1074/jbc.M114.618249
Transmission of atherosclerosis susceptibility with gut microbial transplantation
Abstract
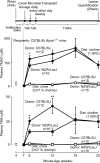
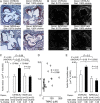
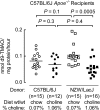
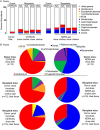
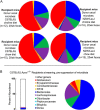
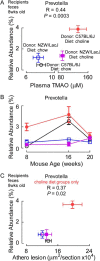
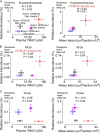
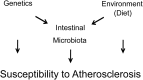
Recent studies indicate both clinical and mechanistic links between atherosclerotic heart disease and intestinal microbial metabolism of certain dietary nutrients producing trimethylamine N-oxide (TMAO). Here we test the hypothesis that gut microbial transplantation can transmit choline diet-induced TMAO production and atherosclerosis susceptibility. First, a strong association was noted between atherosclerotic plaque and plasma TMAO levels in a mouse diversity panel (n = 22 strains, r = 0.38; p = 0.0001). An atherosclerosis-prone and high TMAO-producing strain, C57BL/6J, and an atherosclerosis-resistant and low TMAO-producing strain, NZW/LacJ, were selected as donors for cecal microbial transplantation into apolipoprotein e null mice in which resident intestinal microbes were first suppressed with antibiotics. Trimethylamine (TMA) and TMAO levels were initially higher in recipients on choline diet that received cecal microbes from C57BL/6J inbred mice; however, durability of choline diet-dependent differences in TMA/TMAO levels was not maintained to the end of the study. Mice receiving C57BL/6J cecal microbes demonstrated choline diet-dependent enhancement in atherosclerotic plaque burden as compared with recipients of NZW/LacJ microbes. Microbial DNA analyses in feces and cecum revealed transplantation of donor microbial community features into recipients with differences in taxa proportions between donor strains that were transmissible to recipients and that tended to show coincident proportions with TMAO levels. Proportions of specific taxa were also identified that correlated with plasma TMAO levels in donors and recipients and with atherosclerotic lesion area in recipients. Atherosclerosis susceptibility may be transmitted via transplantation of gut microbiota. Gut microbes may thus represent a novel therapeutic target for modulating atherosclerosis susceptibility.
Keywords: Atherosclerosis; Choline; Dyslipidemia; Gut Microbiota; Koch's Postulate; Lipid; Nutrition; Phospholipid; Trimethylamine N-Oxide (TMAO).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





Similar articles
-
Gut Colonization with Methanogenic Archaea Lowers Plasma Trimethylamine N-oxide Concentrations in Apolipoprotein e-/- Mice.Sci Rep. 2018 Oct 3;8(1):14752. doi: 10.1038/s41598-018-33018-5. Sci Rep. 2018. PMID: 30283097 Free PMC article.
-
Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide.mBio. 2015 Mar 17;6(2):e02481. doi: 10.1128/mBio.02481-14. mBio. 2015. PMID: 25784704 Free PMC article.
-
Berberine attenuates choline-induced atherosclerosis by inhibiting trimethylamine and trimethylamine-N-oxide production via manipulating the gut microbiome.NPJ Biofilms Microbiomes. 2021 Apr 16;7(1):36. doi: 10.1038/s41522-021-00205-8. NPJ Biofilms Microbiomes. 2021. PMID: 33863898 Free PMC article.
-
Gut microbiota in atherosclerosis: focus on trimethylamine N-oxide.APMIS. 2020 May;128(5):353-366. doi: 10.1111/apm.13038. Epub 2020 Mar 30. APMIS. 2020. PMID: 32108960 Free PMC article. Review.
-
Trimethylamine N-Oxide, the Microbiome, and Heart and Kidney Disease.Annu Rev Nutr. 2017 Aug 21;37:157-181. doi: 10.1146/annurev-nutr-071816-064732. Epub 2017 Jul 17. Annu Rev Nutr. 2017. PMID: 28715991 Review.
Cited by
-
Influence of the microbiome on solid organ transplant survival.J Heart Lung Transplant. 2021 Aug;40(8):745-753. doi: 10.1016/j.healun.2021.04.004. Epub 2021 Apr 21. J Heart Lung Transplant. 2021. PMID: 34030971 Free PMC article. Review.
-
Host Genetic Background and Gut Microbiota Contribute to Differential Metabolic Responses to Fructose Consumption in Mice.J Nutr. 2020 Oct 12;150(10):2716-2728. doi: 10.1093/jn/nxaa239. J Nutr. 2020. PMID: 32856048 Free PMC article.
-
Interactions between Gut Microbiota and Natural Bioactive Polysaccharides in Metabolic Diseases: Review.Nutrients. 2024 Aug 24;16(17):2838. doi: 10.3390/nu16172838. Nutrients. 2024. PMID: 39275156 Free PMC article. Review.
-
Gut Microbiome and Colon Cancer: Role of Bacterial Metabolites and Their Molecular Targets in the Host.Curr Colorectal Cancer Rep. 2017 Apr;13(2):111-118. doi: 10.1007/s11888-017-0362-9. Epub 2017 Feb 22. Curr Colorectal Cancer Rep. 2017. PMID: 30337849 Free PMC article.
-
Gut microbiome sheds light on the development and treatment of abdominal aortic aneurysm.Front Cardiovasc Med. 2022 Nov 25;9:1063683. doi: 10.3389/fcvm.2022.1063683. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36505348 Free PMC article. Review.
References
-
- Bäckhed F., Ley R. E., Sonnenburg J. L., Peterson D. A., Gordon J. I. (2005) Host-bacterial mutualism in the human intestine. Science 307, 1915–1920 - PubMed
-
- Wang Z., Klipfell E., Bennett B. J., Koeth R., Levison B. S., Dugar B., Feldstein A. E., Britt E. B., Fu X., Chung Y. M., Wu Y., Schauer P., Smith J. D., Allayee H., Tang W. H., DiDonato J. A., Lusis A. J., Hazen S. L. (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

