Iron therapy in anaemic adults without chronic kidney disease
- PMID: 25550190
- PMCID: PMC10891481
- DOI: 10.1002/14651858.CD010640.pub2
Iron therapy in anaemic adults without chronic kidney disease
Abstract
Background: Anaemia affects about a quarter of the world's population. An estimated 50% of anaemic people have anaemia due to iron deficiency.
Objectives: To assess the safety and efficacy of iron therapies for the treatment of adults with anaemia who are not pregnant or lactating and do not have chronic kidney disease.
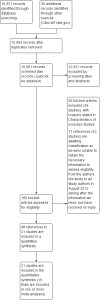
Search methods: We ran the search on 11 July 2013. We searched the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, EMBASE (Ovid SP), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus (EBSCO Host), the Institute for Scientific Information Web of Science (ISI WOS) Scientific Citation Index (SCI)-EXPANDED (1970) and Conference Proceedings Citation Index (CPCI)-Science (1990) and Clinicaltrials.gov; we also screened reference lists. An updated search was run on 24 November 2014 but the results have not yet been incorporated into the review.
Selection criteria: Two review authors independently selected references for further assessment by going through all titles and abstracts. Further selection was based on review of full-text articles for selected references.
Data collection and analysis: Two review authors independently extracted study data. We calculated the risk ratio (RR) with 95% confidence interval (CI) for binary outcomes and the mean difference (MD) or the standardised mean difference (SMD) with 95% CI for continuous outcomes. We performed meta-analysis when possible, when I(2) was less than or equal to 80% using a fixed-effect or random-effects model, using Review Manager software. The range of point estimates for individual studies is presented when I(2) > 80%.
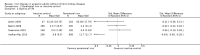
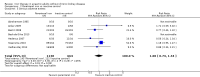
Main results: We included in this systematic review 4745 participants who were randomly assigned in 21 trials. Trials were conducted in a wide variety of clinical settings. Most trials included participants with mild to moderate anaemia and excluded participants who were allergic to iron therapy. All trials were at high risk of bias for one or more domains. We compared both oral iron and parenteral iron versus inactive controls and compared different iron preparations.The comparison between oral iron and inactive control revealed no evidence of clinical benefit in terms of mortality (RR 1.05, 95% CI 0.68 to 1.61; four studies, N = 659; very low-quality evidence). The point estimate of the mean difference in haemoglobin levels in individual studies ranged from 0.3 to 3.1 g/dL higher in the oral iron group than in the inactive control group. The proportion of participants who required blood transfusion was lower with oral iron than with inactive control (RR 0.74, 95% CI 0.55 to 0.99; three studies, N = 546; very low-quality evidence). Evidence was inadequate for determination of the effect of parenteral iron on mortality versus oral iron (RR 1.49, 95% CI 0.56 to 3.94; 10 studies, N = 2141; very low-quality evidence) or inactive control (RR 1.04, 95% CI 0.63 to 1.69; six studies, N = 1009; very low-quality evidence). Haemoglobin levels were higher with parenteral iron than with oral iron (MD -0.50 g/dL, 95% CI -0.73 to -0.27; six studies, N = 769; very low-quality evidence). The point estimate of the mean difference in haemoglobin levels in individual studies ranged between 0.3 and 3.0 g/dL higher in the parenteral iron group than in the inactive control group. Differences in the proportion of participants requiring blood transfusion between parenteral iron and oral iron groups (RR 0.61, 95% CI 0.24 to 1.58; two studies, N = 371; very low-quality evidence) or between parenteral iron groups and inactive controls (RR 0.84, 95% CI 0.66 to 1.06; eight studies, N = 1315; very low-quality evidence) were imprecise. Average blood volume transfused was less in the parenteral iron group than in the oral iron group (MD -0.54 units, 95% CI -0.96 to -0.12; very low-quality evidence) based on one study involving 44 people. Differences between therapies in quality of life or in the proportion of participants with serious adverse events were imprecise (very low-quality evidence). No trials reported severe allergic reactions due to parenteral iron, suggesting that these are rare. Adverse effects related to oral iron treatment included nausea, diarrhoea and constipation; most were mild.Comparisons of one iron preparation over another for mortality, haemoglobin or serious adverse events were imprecise. No information was available on quality of life. Thus, little evidence was found to support the use of one preparation or regimen over another.Subgroup analyses did not reveal consistent results; therefore we were unable to determine whether iron is useful in specific clinical situations, or whether iron therapy might be useful for people who are receiving erythropoietin.
Authors' conclusions: • Very low-quality evidence suggests that oral iron might decrease the proportion of people who require blood transfusion, and no evidence indicates that it decreases mortality. Oral iron might be useful in adults who can tolerate the adverse events, which are usually mild.• Very low-quality evidence suggests that intravenous iron results in a modest increase in haemoglobin levels compared with oral iron or inactive control without clinical benefit.• No evidence can be found to show any advantage of one iron preparation or regimen over another.• Additional randomised controlled trials with low risk of bias and powered to measure clinically useful outcomes such as mortality, quality of life and blood transfusion requirements are needed.
Conflict of interest statement
KSG: none known.
MN: none known.
JFB: none known.
SDA: (in part through employer) has received grants and fees for lectures, consultancy and/or board membership from Abbott Vascular, Amgen, Bayer, BG Medicine, Bioventrix, Brahms, Cardiomems, Novartis, BG Medicine, Impulse Dynamics, Medical Sensible, Pluristem, Psioxus, Relypsa, Servier and/or Vifor.
TR: UCL received an educational grant from Vifor Pharma in 2009. This funded a research fellow who undertook an audit of anaemia in surgical patients. These data have been presented at national and international meetings. TR is currently Chief Investigator for an NIHR HTA trial that is being conducted to assess the role of Intravenous iron in the treatment of individuals with anaemia before major surgery (PREVENTT). No funding was provided for this Cochrane review, and no input was received from anyone other than study authors. TR received travel funds from the BBTS, NATA, Pharmocosmos, Vifor Pharma and other industry.
Figures






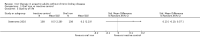
















Update of
- doi: 10.1002/14651858.CD010640
References
References to studies included in this review
Abrahamsen 1965 {published data only}
-
- Abrahamsen AF. The effect of orally and parenterally administered iron in posthaemorrhagic anaemia. Acta Medica Scandinavica 1965;177(4):503‐7. - PubMed
Anker 2009 {published data only}
-
- Anker SD, Colet JC, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. Ferric carboxymaltose assessment in patients with Iron deficiency and chronic heart failure with and without anemia (FAIR‐HF): a randomized, double‐blind, placebo‐controlled, international multi‐center phase III study. Circulation 2010;120(21):2156.
-
- Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. New England Journal of Medicine 2009;361(25):2436‐48. - PubMed
-
- Comin‐Colet J, Lainscak M, Dickstein K, Filippatos G, Johnson P, Luscher TF, et al. Influence of intravenous ferric carboxymaltose on health‐related quality of life measures in patients with chronic heart failure and iron deficiency: an analysis from the FAIR‐HF study. European Journal of Heart Failure Supplements 2010;9:S120. - PMC - PubMed
-
- Comin‐Colet J, Lainscak M, Dickstein K, Filippatos G, Johnson P, Luscher TF, et al. Intravenous ferric carboxymaltose improves health‐related quality of life in patients with chronic heart failure and iron deficiency irrespective of renal function and anaemia status: an analysis from the FAIR‐HF study. NDT Plus 2010;3(3 Suppl 3):iii217.
-
- Comin‐Colet J, Lainscak M, Dickstein K, Filippatos GS, Johnson P, Luscher TF, et al. The effect of intravenous ferric carboxymaltose on health‐related quality of life in patients with chronic heart failure and iron deficiency: a subanalysis of the FAIR‐HF study. European Heart Journal 2012; Vol. Epub ahead of print. - PMC - PubMed
Auerbach 2010 {published data only}
-
- Auerbach M, Silberstein PT, Webb RT, Averyanova S, Ciuleanu TE, Cam L, et al. Darbepoetin alfa (DA) 500MCG or 300MCG once every three weeks (Q3W) with or without iron in patients (PTS) with chemotherapy‐induced anemia (CIA). Annals of Oncology 2008;19(S8):viii3.
-
- Auerbach M, Silberstein PT, Webb RT, Averyanova S, Ciuleanu TE, Shao J, et al. Darbepoetin alfa 300 or 500 μg once every 3 weeks with or without intravenous iron in patients with chemotherapy‐induced anemia. American Journal of Hematology 2010;85(9):655‐63. - PubMed
Bastit 2008 {published data only}
-
- Altintas S, Bastit L, Vandebroek A, Mossman T, Suto T. Analysis of quality of life responses by efficacy response status in cancer patients with chemotherapy‐induced anaemia who received darbopoetin alfa 500 mcg every 3 weeks and IV iron [abstract]. Haematologica 2007;97(Suppl 1):286‐7.
-
- Bastit L, Vandebroek A, Altintas S, Gaede B, Pinter T, Suto TS, et al. Randomized, multicenter, controlled trial comparing the efficacy and safety of darbepoetin alpha administered every 3 weeks with or without intravenous iron in patients with chemotherapy‐induced anemia. Journal of Clinical Oncology 2008;26(10):1611‐8. - PubMed
-
- Lerchenmueller C, Husseini F, Gaede B, Mossman T, Suto T, Vanderbroek A. Intravenous (IV) iron supplementation in patients with chemotherapy‐induced anemia (CIA) receiving darbepoetin alfa every 3 weeks (Q3W): iron parameters in a randomized controlled trial. Blood 2006;108(11):Abstract 1552.
-
- Pinter T, Mossman T, Suto T, Vansteenkiste J. Effects of intravenous (IV) iron supplementation on responses to every‐3‐week (Q3W) darbepoetin alfa (DA) by baseline hemoglobin in patients (pts) with chemotherapy‐induced anemia (CIA) [abstract]. Journal of Clinical Oncology 2007;25(18S Part I):519.
-
- Vandebroek A, Altintas S, Gaede B, Smith K, Yao B, Schupp M. Darbepoetin alfa administered every 3 weeks with or without parenteral iron in anaemic patients with nonmyeloid malignancies receiving chemotherapy: interim results from a randomised open‐label study. Haematologica 2006;91(Suppl 1):12.
Beck‐da‐Silva 2013 {published data only}
-
- Beck‐da‐Silva L, Piardi D, Soder S, Rohde LE, Pereira‐Barretto AC, Albuquerque D, et al. IRON‐HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. International Journal of Cardiology 2013; Vol. Epub ahead of print. - PubMed
-
- Beck‐da‐Silva L, Rohde LE, Pereira‐Barretto AC, Albuquerque D, Bocchi E, Vilas‐Boas F, et al. Rationale and design of the IRON‐HF study: a randomized trial to assess the effects of iron supplementation in heart failure patients with anemia. Journal of Cardiac Failure 2007;13(1):14‐7. - PubMed
Dangsuwan 2010 {published data only}
-
- Dangsuwan P, Manchana T. Blood transfusion reduction with intravenous iron in gynecologic cancer patients receiving chemotherapy. Gynecologic Oncology 2010;116(3):522‐5. - PubMed
Edwards 2009 {published data only}
-
- Edwards TJ, Noble EJ, Durran A, Mellor N, Hosie KB. Randomized clinical trial of preoperative intravenous iron sucrose to reduce blood transfusion in anaemic patients after colorectal cancer surgery. The British Journal of Surgery 2009;96(10):1122‐8. - PubMed
-
- Noble E, Edwards T, Durran A, Mellor N, Hosie KB. A prospective blinded placebo controlled randomised trial of intravenous iron supplementation in patients undergoing colorectal cancer surgery. Colorectal Disease 2009;11(Suppl S1):31.
Evstatiev 2011 {published data only}
-
- Evstatiev R, Marteau P, Iqbal T, Khalif I, Gudehus M, Gasche C. Intravenously administered ferric carboxymaltose and iron sucrose significantly improve quality of life in patients with IBD‐associated iron deficiency anaemia. Journal of Crohn's and Colitis 2011;5(1):S91.
-
- Evstatiev R, Marteau P, Iqbal T, Khalif IL, Stein J, Bokemeyer B, et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology 2011;141(3):846‐53 e1‐2. - PubMed
-
- Iqbal T, Evstatiev R, Chopey K, Harjunpaa J, Gasche C. Significant benefit of ferric carboxymaltose in IBD‐associated iron deficiency anaemia is independent of patient baseline characteristics. Journal of Crohn's and Colitis 2011;5(1):S96‐7.
Hedenus 2007 {published data only}
-
- Hedenus M, Birgegard G, Nasman P, Ahlberg L, Karlsson T, Lauri B, et al. Addition of intravenous iron to epoetin beta increases hemoglobin response and decreases epoetin dose requirement in anemic patients with lymphoproliferative malignancies: a randomized multicenter study. Leukemia 2007;21(4):627‐32. - PubMed
-
- Hedenus M, Birgegard G, Nasman P, Ahlberg L, Karlsson T, Lauri B, et al. Erratum: Addition of intravenous iron to epoetin beta increases hemoglobin response and decreases epoetin dose requirement in anemic patients with lymphoproliferative malignancies: a randomized multicenter study (Leukemia (2007) vol 21 (627‐32) 10.1038/sj.leu.2404562). Leukemia 2008;22(2):462. - PubMed
-
- Hedenus M, Birgegard G, Näsman P, Ahlberg L, Karlsson T, Lauri B, et al. Adjuvant intravenous iron therapy potentiates epoetin beta treatment in anemic, non iron‐depleted patients with lymphoproliferative disorders: results of the NIFE study. Hematologica 2006;91(Suppl 1):365.
Hetzel 2012 {published data only}
-
- Hetzel D, Strauss W, Bernard K, Li J, Allen L. IV iron treatment of iron deficiency anaemia with ferumoxytol in patients with gastrointestinal disorders unable to take oral iron: a randomized controlled trial versus iron sucrose. Journal of Crohn's and Colitis 2013;7(Suppl 1):S204.
-
- Hetzel D, Urboniene A, Bernard K, Strauss W, Cressman M, Li Z, et al. Potential new treatment option for iron deficiency anemia patients with a history of unsatisfactory oral iron therapy: results of a phase III, randomized, open‐label, active‐controlled trial of ferumoxytol. Blood 2012;120(21):2009.
Karkouti 2006 {published data only}
-
- Karkouti K, McCluskey SA, Ghannam M, Salpeter MJ, Quirt I, Yau TM. Intravenous iron and recombinant erythropoietin for the treatment of postoperative anemia. Canadian Journal of Anaesthesia 2006;53(1):11‐9. - PubMed
Lidder 2007 {published data only}
-
- Lidder PG, Hosie KB, Marsden M. A double blind prospective randomized controlled trial of iron supplementation in anaemic patients scheduled for colorectal cancer surgery. Colorectal Disease 2004;6(Suppl 1):52‐3.
Lindgren 2009 {published data only}
-
- Lindgren S, Wikman O, Befrits R, Blom H, Eriksson A, Granno C, et al. Intravenous iron sucrose is superior to oral iron sulphate for correcting anaemia and restoring iron stores in IBD patients: a randomized, controlled, evaluator‐blind, multicentre study. Scandanavian Journal of Gastroenterology 2009;44(7):838‐45. - PubMed
Maccio 2010 {published data only}
-
- Maccio A, Madeddu C, Gramignano G, Mulas C, Sanna E, Mantovani G. Efficacy and safety of oral lactoferrin supplementation associated with rHuEPObeta for the treatment of anemia in advanced cancer patients submitted to chemotherapy. Journal of Clinical Oncology 2010;28(15 Suppl 1):2010. - PMC - PubMed
-
- Maccio A, Madeddu C, Gramignano G, Mulas C, Sanna E, Mantovani G. Efficacy and safety of oral lactoferrin supplementation in combination with rHuEPO‐beta for the treatment of anemia in advanced cancer patients undergoing chemotherapy: open‐label, randomized controlled study. Oncologist 2010;15(8):894‐902. - PMC - PubMed
Madi‐Jebara 2004 {published data only}
-
- Madi‐Jebara SN, Sleilaty GS, Achouh PE, Yazigi AG, Haddad FA, Hayek GM, et al. Postoperative intravenous iron used alone or in combination with low‐dose erythropoietin is not effective for correction of anemia after cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 2004;18(1):59‐63. - PubMed
Parker 2010 {published data only}
-
- Parker MJ. Iron supplementation for anemia after hip fracture surgery: a randomized trial of 300 patients. The Journal of Bone and Joint Surgery. American Volume 2010;92(2):265‐9. - PubMed
Pieracci 2009 {published data only}
-
- Pieracci FM, Henderson P, Rodney JR, Holena DN, Genisca A, Ip I, et al. Randomized, double‐blind, placebo‐controlled trial of effects of enteral iron supplementation on anemia and risk of infection during surgical critical illness. Surgical Infections 2009;10(1):9‐19. - PubMed
Steensma 2010 {published data only}
-
- Steensma DP, Dakhil SR, Dalton R, Kahanic SP, Kugler JW, Stella PJ, et al. A phase III, randomized study of the effects of parenteral iron, oral iron, or no iron supplementation on the erythropoietic response to darbepoetin alfa for patients with chemotherapy‐associated anemia: a study of the Mayo Clinic Cancer Research Consortium (MCCRC). Blood 2009;114(22):Abstract 630. - PMC - PubMed
-
- Steensma DP, Sloan JA, Dakhil SR, Dalton R, Kahanic SP, Prager DJ, et al. Phase III, randomized study of the effects of parenteral iron, oral iron, or no iron supplementation on the erythropoietic response to darbepoetin alfa for patients with chemotherapy‐associated anemia. Journal of Clinical Oncology 2010;29(1):97‐105. - PMC - PubMed
Sutton 2004 {published data only}
-
- Sutton PM, Cresswell T, Livesey JP, Speed K, Bagga T. Treatment of anaemia after joint replacement: a double‐blind, randomised, controlled trial of ferrous sulphate versus placebo. The Journal of Bone and Joint Surgery. British Volume 2004;86(1):31‐3. - PubMed
Vadhan‐Raj 2013 {published data only}
-
- Dickerhoof R, DeBusk K, Bernard K, Strauss W, Allen LF, Acaster S. Measuring fatigue in iron deficiency anemia patients: a psychometric validation study. Value in Health 2013;16(3):A36.
-
- Raj SV, Cressman M, Ford D, Strauss W, Poss G, Bernard K, et al. Ferumoxytol treatment results in robust hemoglobin increases in iron deficiency anemia patients with a history of unsatisfactory oral iron therapy in a phase III, randomized, placebo‐controlled trial. Blood 2012;120(21):2098.
-
- Raj SV, Hsieh A, Strauss W, Stevenson M, Bernard K, Allen LF. Ferumoxytol treatment demonstrates significant improvements in fatigue and health‐related quality of life in iron deficiency anemia patients with a history of unsatisfactory oral iron therapy. Blood 2012;120(21):478.
Van Wyck 2009 {published data only}
-
- Wyck DB, Mangione A, Morrison J, Hadley PE, Jehle JA, Goodnough LT. Large‐dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion 2009;49(12):2719‐28. - PubMed
References to studies excluded from this review
Anker 2006 {published data only}
-
- Anker SD, Okonko DO, Grzeslo A, Witkowski T, Missouris CG, Banasiak W, et al. Intravenous iron sucrose in anemic and non‐anemic iron deficient patients with CHF: a randomized, controlled, observer‐blinded intervention study (FERRIC‐HF). Journal of Cardiac Failure 2006;12(8):S179.
Anker 2009a {published data only}
-
- Anker SD, Colet JC, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. Rationale and design of Ferinject((R)) Assessment in patients with IRon deficiency and chronic Heart Failure (FAIR‐HF) study: a randomized, placebo‐controlled study of intravenous iron supplementation in patients with and without anaemia. European Journal of Heart Failure 2009;11(11):1084‐91. - PMC - PubMed
Anonymous 1966 {published data only}
-
- Anonymous. The therapeutic effectiveness of various compounds containing iron. Nutrition Reviews 1966;24(8):232‐5. - PubMed
Aronstam 1982 {published data only}
-
- Aronstam A, Aston DL. A comparative trial of a controlled‐release iron tablet preparation ('Ferrocontin' Continus) and ferrous fumarate tablets. Pharmatherapeutica 1982;3(4):263‐7. - PubMed
Auerbach 2007 {published data only}
-
- Auerbach M, Henry DH. Increased importance of intravenous iron in chemotherapy‐induced anemia. Journal of Clinical Oncology 2007;25(15):2145‐6. - PubMed
Auerbach 2011 {published data only}
-
- Auerbach M, Pappadakis JA, Bahrain H, Auerbach SA, Ballard H, Dahl NV. Safety and efficacy of rapidly administered (one hour) one gram of low molecular weight iron dextran (INFeD) for the treatment of iron deficient anemia. American Journal of Hematology 2011;86(10):860‐2. - PubMed
Barish 2012 {published data only}
Barrison 1981 {published data only}
Beris 2006 {published data only}
-
- Beris P, Verholen F, Sadowski M, Noger M, Hoffmeyer P. Correction of anemia of the post‐operative period after orthopedic surgery by oral versus intravenous iron versus intravenous iron epo: a prospective randomized trial. Haematologica 2006;91(Suppl 1):6.
Bermejo 2009 {published data only}
-
- Bermejo San Jose F. [Is intravenous iron really useful in inflammatory bowel disease? Would oral iron not be simpler and cheaper?]. Gastroenterologia y Hepatologia 2009;32(1):63‐4. - PubMed
Bernabeu‐Wittel 2012 {published data only}
-
- Bernabeu‐Wittel M, Aparicio R, Romero M, Murcia‐Zaragoza J, Monte‐Secades R, Rosso C, et al. Ferric carboxymaltose with or without erythropoietin for the prevention of red‐cell transfusions in the perioperative period of osteoporotic hip fractures: a randomized controlled trial. The PAHFRAC‐01 project. BMC Musculoskeletal Disorders 2012;13:27 (1 to 8). - PMC - PubMed
Bisbe 2011 {published data only}
-
- Bisbe E, Garcia‐Erce JA, Diez‐Lobo AI, Munoz M. A multicentre comparative study on the efficacy of intravenous ferric carboxymaltose and iron sucrose for correcting preoperative anaemia in patients undergoing major elective surgery. British Journal of Anaesthesia 2011;107(3):477‐8. - PubMed
Black 1981 {published data only}
-
- Black GS. A comparison of two iron tablet preparations in the treatment of iron deficiency anaemia. Journal of International Medical Research 1981;9(4):295‐6. - PubMed
Bulvik 1997 {published data only}
-
- Bulvik S, Karalnick S, Leibovitz G, Benist A, Niven M. Intravenous iron gluconate or iron saccharate for patients with iron deficiency anemia with malabsorption or oral iron intolerance. Blood 1997;90(10 Suppl 1 Pt 2):11b.
Crosby 1994 {published data only}
-
- Crosby L, Palarski VA, Cottington E, Cmolik B. Iron supplementation for acute blood loss anemia after coronary artery bypass surgery: a randomized, placebo‐controlled study. Heart & Lung 1994;23(6):493‐9. - PubMed
Cuenca 2008 {published data only}
-
- Cuenca J, Garcia‐Erce JA, Munoz M. Efficacy of intravenous iron sucrose administration for correcting preoperative anemia in patients scheduled for major orthopedic surgery. Anesthesiology 2008;109(1):151‐2. - PubMed
Desai 1968 {published data only}
Earley 2009 {published data only}
Evers 1977 {published data only}
-
- Evers JEM. Iron‐poly(sorbitol‐gluconic acid) complex and iron‐dextran in the treatment of severe iron deficiency anaemia. Scandinavian Journal of Haematology 1977;19(Suppl 32):279‐85. - PubMed
Evstatiev 2013 {published data only}
-
- Evstatiev R, Alexeeva O, Bokemeyer B, Chopey I, Felder M, Gudehus M, et al. Ferric carboxymaltose prevents recurrence of anemia in patients with inflammatory bowel disease. Clinical Gastroenterology and Hepatology 2013;11(3):269‐77. - PubMed
Fitzgerald 2008 {published data only}
-
- Fitzgerald JE, Simpson JA, Acheson AG. The use of intravenous iron in patients with cancer related anaemia: don't overlook iron deficiency anaemia in colorectal cancer. British Journal of Haematology 2008;143(5):754. - PubMed
Froessler 2010 {published data only}
-
- Froessler B, Papendorf D. Intravenous iron sucrose—an effective and attractive modality for perioperative anaemia management. Anaesthesia and Intensive Care 2010;38(5):960‐2. - PubMed
Garrido‐Martin 2012 {published data only}
-
- Garrido‐Martin P, Nassar‐Mansur MI, Llana‐Ducros R, Virgos‐Aller TM, Fortunez PMR, Avalos‐Pinto R, et al. The effect of intravenous and oral iron administration on perioperative anaemia and transfusion requirements in patients undergoing elective cardiac surgery: a randomized clinical trial. Interactive CardioVascular and Thoracic Surgery 2012;15(6):1013‐8. - PMC - PubMed
Gasche 1995 {published data only}
-
- Gasche C, Dejaco C, Waldhor T, Reinisch W, Tillinger W, Vogelsang H, et al. [Double‐blind, placebo‐controlled study of erythropoietin and iron saccharate in the treatment of anaemia in Crohn's disease]. Zeitschrift fur Gastroenterologie 1995;33(5):320.
Gedik 1995 {published data only}
-
- Gedik Y, Erduran E, Mocan H, Aynaci FM, Okten A. The efficacy of ferrous sulfate and ferric polymaltose in therapy of iron deficiency anemia. Annals of Medical Sciences 1995;4(2):143‐4.
Grote 2009 {published data only}
-
- Grote L, Leissner L, Hedner J, Ulfberg J. A randomized, double‐blind, placebo controlled, multi‐center study of intravenous iron sucrose and placebo in the treatment of restless legs syndrome. Movement Disorders 2009;24(10):1445‐52. - PubMed
Harris 2009 {published data only}
-
- Harris S, Tepper D, Ip R. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency‐ferric‐HF: a randomized, controlled, observer‐blinded trial. Congestive Heart Failure 2009;15(4):208. - PubMed
Hussain 2013 {published data only}
Kaltwasser 1987 {published data only}
-
- Kaltwasser JP, Ockelmann R, Schalk KP. Oral iron therapy: preparations with rapid or delayed iron liberation?. Medizinische Klinik 1987;82(21):730‐5. - PubMed
Kaltwasser 1989 {published data only}
-
- Kaltwasser JP, Schwarz‐Van d SW. Oral iron treatment: bioavailability and therapeutic efficacy of ferrous iron as effervescent tablets in posthaemorrhagic iron‐deficiency anaemia. Deutsche Medizinische Wochenschrift 1989;114(31‐32):1188‐95. - PubMed
Kulnigg 2009 {published data only}
-
- Kulnigg S, Teischinger L, Dejaco C, Waldhor T, Gasche C. Rapid recurrence of IBD‐associated anemia and iron deficiency after intravenous iron sucrose and erythropoietin treatment. American Journal of Gastroenterology 2009;104(6):1460‐7. - PubMed
Kulnigg‐Dabsch 2012 {published data only}
Lewinski 1973 {published data only}
-
- Lewinski U, Lijn E, Djaldetti M. [Intravenous iron‐dextran versus peroral iron and blood transfusion for anemia]. Harefuah 1973;85(4):167‐9. - PubMed
Liguori 1993 {published data only}
-
- Liguori L. Iron protein succinylate in the treatment of iron deficiency: controlled, double‐blind, multicenter clinical trial on over 1,000 patients. International journal of Clinical Pharmacology, Therapy, and Toxicology 1993;31(3):103‐23. - PubMed
Lipsic 2010 {published data only}
-
- Lipsic E, Meer P. Erythropoietin, iron, or both in heart failure: FAIR‐HF in perspective. European Journal of Heart Failure 2010;12(2):104‐5. - PubMed
Littlewood 2012 {published data only}
-
- Littlewood TJ. Intravenous or oral iron?. American Journal of Hematology 2012;87(2):134‐5. - PubMed
Liu 2004 {published data only}
-
- Liu TC, Lin SF, Chang CS, Yang WC, Chen TP. Comparison of a combination ferrous fumarate product and a polysaccharide iron complex as oral treatments of iron deficiency anemia: a Taiwanese study. International Journal of Hematology 2004;80(5):416‐20. - PubMed
Mundy 2005 {published data only}
-
- Mundy GM, Birtwistle SJ, Power RA. The effect of iron supplementation on the level of haemoglobin after lower limb arthroplasty. The Journal of Bone and Joint Surgery. British Volume 2005;87(2):213‐7. - PubMed
Munoz 2011 {published data only}
-
- Munoz M, Naveira E, Seara J, Cordero J, Marcos F, Bisbe E. Postoperative intravenous iron after lower limb arthroplasty: a comparative study of different doses on transfusion requirements. European Journal of Anaesthesiology 2011;28:90.
Munoz 2011a {published data only}
-
- Munoz M, Naveira E, Seara J, Cordero J, Martos F. Postoperative iron after lower limb arthroplasty: a comparative study of the effects of 300 vs. 600 mg IV iron administration on transfusion requirements. Transfusion Alternatives in Transfusion Medicine 2011;12(1):34‐5.
Munoz 2012 {published data only}
-
- Munoz M, Naveira E, Seara J, Cordero J. Effects of postoperative intravenous iron on transfusion requirements after lower limb arthroplasty. British Journal of Anaesthesia 2012;108(3):532‐4. - PubMed
Murgel 1969 {published data only}
-
- Murgel GA. Therapy of hypochromic anemia using ferrous sulfate (comparative study of reinforced sustained‐release pills and conventional pills). Revista Brasileira de Medicina 1969;26(6):375‐9. - PubMed
Najean 1995 {published data only}
-
- Najean Y, Acuto G, Scotti A. Multicentre double‐blind clinical trial of iron protein succinylate in comparison with iron sulfate in the treatment of iron deficiency anaemia. Clinical Drug Investigation 1995;10(4):198‐207.
Onken 2013 {published data only}
-
- Bregman DB, Morris D, Koch TA, He A, Goodnough LT. Hepcidin levels predict nonresponsiveness to oral iron therapy in patients with iron deficiency anemia. American Journal of Hematology 2013;88(2):97‐101. - PubMed
-
- Onken JE, Bregman DB, Harrington RA, Morris D, Acs P, Akright B, et al. A multicenter, randomized, active‐controlled study to investigate the efficacy and safety of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Transfusion 2013; Vol. Epub ahead of print. - PubMed
Ravanbod 2013 {published data only}
-
- Ravanbod M, Asadipooya K, Kalantarhormozi M, Nabipour I, Omrani GR. Treatment of iron‐deficiency anemia in patients with subclinical hypothyroidism. American Journal of Medicine 2013;126(5):420‐4. - PubMed
Raya 2010 {published data only}
-
- Raya J, Garrido P, Pecos P, Martinez R, Nassar I, Llana R, et al. Analysis of two different schemes of iron treatment to improve postoperative anemia in cardiac surgery: a randomized double‐disguised, triple‐blind study. Haematologica 2010;95(Suppl 2):706.
Rondinelli 2013 {published data only}
-
- Rondinelli MB, Inghilleri G, Pavesi M, Bartolomei A, Pagnotta R, Terlizzi F, et al. Efficacy of ferrous bisglycinate for the management of patient undergoing pre‐operative blood donation or with pre‐operative anaemia in orthopedic surgery: a prospective study. Transfusion Medicine 2013;23(s1):36.
Schatz 2013 {published data only}
-
- Schatz U, Arneth B, Siegert G, Siegels D, Fischer S, Julius U, et al. Iron deficiency and its management in patients undergoing lipoprotein apheresis. Comparison of two parenteral iron formulations. Atherosclerosis Supplements 2013;14(1):115‐22. - PubMed
Serrano‐Trenas 2011 {published data only}
-
- Serrano‐Trenas JA, Ugalde PF, Cabello LM, Chofles LC, Lazaro PS, Benitez PC. Role of perioperative intravenous iron therapy in elderly hip fracture patients: a single‐center randomized controlled trial. Transfusion 2011;51(1):97‐104. - PubMed
Singh 2007 {published data only}
-
- Singh A, Hertel J, Bernardo M, Baptista J, Kausz A, Brenner L, et al. A double‐blind, placebo‐controlled, randomized phase III study of the safety of ferumoxytol as a new intravenous iron replacement therapy. American Journal of Kidney Diseases 2007;49(4):A32.
Strauss 2013 {published data only}
-
- Strauss W, Bernard K, Li Z, Allen L. Safety and efficacy of ferumoxytol vs. iron sucrose in the treatment of iron deficiency anemia (IDA) in patients with normal and abnormal renal function. Nephrology Dialysis Transplantation 2013;28(Suppl 1):i9.
Wang 2003 {published data only}
-
- Wang L, Li G, Liao C, Wang F. The effects of oral vs venous iron supplement in treatment of iron deficiency of maintained patients with anemia [Abstract No. PUB324]. Journal of the American Society of Nephrology 2003;14(Suppl S):842a.
Weatherall 2004 {published data only}
-
- Weatherall M, Maling TJ. Oral iron therapy for anaemia after orthopaedic surgery: randomized clinical trial. ANZ Journal of Surgery 2004;74(12):1049‐51. - PubMed
Zauber 1992 {published data only}
-
- Zauber NP, Zauber AG, Gordon FJ, Tillis AC, Leeds HC, Berman E, et al. Iron supplementation after femoral head replacement for patients with normal iron stores. JAMA 1992;267(4):525‐7. - PubMed
References to studies awaiting assessment
Adsul 2005 {published data only}
-
- Adsul BB, Desai A, Gawde A, Baliga V. Comparative assessment of the bioavailability, efficacy and safety of a modified‐release (MR) carbonyl iron tablet and oral conventional iron preparation in adult Indian patients with nutritional iron deficiency anaemia. Journal of the Indian Medical Association 2005;103(6):338‐42. - PubMed
Anthony 2011 {published data only}
-
- Anthony LB, Gabrail NY, Ghazal H, Woytowitz DV, Flam MS, Drelichman A, et al. IV iron sucrose for cancer and/or chemotherapy‐induced anemia in patients treated with erythropoiesis stimulating agents. Community Oncology 2011;8(6):270‐8.
-
- Bellet RE, Ghazal H, Flam M, Drelichman A, Gabrail N, Woytowitz D, et al. A phase III randomized controlled study comparing iron sucrose intravenously (IV) to no iron treatment of anemia in cancer patients undergoing chemotherapy and erythropoietin stimulating agent (ESA) therapy [Abstract No. 9109]. Journal of Clinical Oncology 2007;25(18S Part I):519.
Auerbach 2004 {published data only}
-
- Auerbach M, Ballard H, Trout JR, McIlwain M, Ackerman A, Bahrain H, et al. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy‐related anemia: a multicenter, open‐label, randomized trial. Journal of Clinical Oncology 2004;22(7):1301‐7. - PubMed
-
- Auerbach M, Barker L, Bahrain H, Trout R, McIlwain M, Ballard H. Intravenous iron (IV Fe) optimizes the response to erythropoietin (EPO) in patients with anemia of cancer and cancer chemotherapy: results of a multicenter, open‐label, randomized trial. Blood 2001;98(11):799A. - PubMed
-
- Ballard H, Rana J, Ackerman A, Merino R, Rosenoff S, Kastritsis C, et al. Total dose infusion (TDI) of iron dextran (ID) optimizes erythropoietin (EPO) responsiveness in the anemia of cancer (CA) [abstract]. Proceedings of the American Society of Clinical Oncology 1999;18:581a.
Bisbe 2012 {published data only}
-
- Bisbe E. Intravenous iron to treat post‐operative anaemia. Transfusion Medicine 2013;23(s1):13‐4.
-
- Bisbe E. Intravenous iron to treat postoperative anaemia. Vox Sanguinis 2013;105(Suppl 1):50.
-
- Bisbe E, Molto L, Arroyo R, Santiveri X, Munoz M. The efficacy of intravenous iron for treating postoperative anemia and hastening functional recovery in patients undergoing total knee arthroplasty: preliminary results from a randomized controlled trial. Transfusion Alternatives in Transfusion Medicine 2012;12(2):29‐30.
Chen 2002 {published data only}
-
- Chen BB, Lin GW, Wu W, Chen Y, Wang JE, Wang YL. Comparitive study on three iron preparations for treatment of iron deficiency anemia: a randomized‐controlled trial. Shanghai Medical Journal 2002;25(3):154‐7.
Devasthali 1991 {published data only}
-
- Devasthali SD, Gordeuk VR, Brittenham GM, Bravo JR, Hughes MA, Keating LJ. Bioavailability of carbonyl iron: a randomized, double‐blind study. European Journal of Haematology 1991;46(5):272‐8. - PubMed
Erichsen 2005 {published data only}
-
- Erichsen K, Ulvik RJ, Grimstad T, Berstad A, Berge RK, Hausken T. Effects of ferrous sulphate and non‐ionic iron‐polymaltose complex on markers of oxidative tissue damage in patients with inflammatory bowel disease. Alimentary Pharmacology & Therapeutics 2005;22(9):831‐8. - PubMed
-
- Erichsen K, Ulvik RJ, Nysaeter G, Johansen J, Ostborg J, Berstad A, et al. Oral ferrous fumarate or intravenous iron sucrose for patients with inflammatory bowel disease. Scandinavian Journal of Gastroenterology 2005;40(9):1058‐65. - PubMed
Ferrari 2012 {published data only}
-
- Ferrari P, Nicolini A, Manca ML, Rossi G, Anselmi L, Conte M, et al. Treatment of mild non‐chemotherapy‐induced iron deficiency anemia in cancer patients: comparison between oral ferrous bisglycinate chelate and ferrous sulfate. Biomedicine and Pharmacotherapy 2012; Vol. Epub ahead of print. - PubMed
Giordano 2011 {published data only}
-
- Giordano G, Mondello P, Tambaro R, Maria M, D'Amico F, Sticca G, et al. Intravenous iron support vs oral liposomal iron support in patients with refractory anemia treated with Epo alpha. Monocentric prospective study. Leukemia Research 2011;35:S137.
Gordeuk 1987 {published data only}
-
- Gordeuk VR, Brittenham GM, Hughes M, Keating LJ, Opplt JJ. High‐dose carbonyl iron for iron deficiency anemia: a randomized double‐blind trial. The American Journal of Clinical Nutrition 1987;46(6):1029‐34. - PubMed
Henry 2007 {published data only}
-
- Henry DH, Dahl NV. Does quality of life improvement precede anemia correction in patients with chemotherapy‐induced anemia treated with intravenous iron? [abstract]. Journal of Clinical Oncology 2007;25(18S Part I):513.
-
- Henry DH, Dahl NV, Auerbach M, Tchekmedyian S, Laufman LR. Intravenous ferric gluconate (FG) for increasing response to epoetin (EPO) in patients with anemia of cancer chemotherapy: results of a multicenter, randomized trial. Blood 2004;104(11 Part 2):10b.
-
- Henry DH, Dahl NV, Auerbach M, Tchekmedyian S, Laufman LR. Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist 2007;12(2):231‐42. - PubMed
Izuel‐Rami 2006 {published data only}
-
- Izuel‐Rami M, Garcia‐Erce JA, Cuenca J, Gomez‐Barrera M, Villar I, Rabanaque MJ. Recovery from postoperative anaemia after hip fracture replacement surgery. Is there a role for erythropoietin and intravenous iron?. Transfusion Alternatives in Transfusion Medicine 2006;8(1 Suppl):81.
Jacobs 2000 {published data only}
-
- Jacobs P, Wood L, Bird AR. Erythrocytes: better tolerance of iron polymaltose complex compared with ferrous sulphate in the treatment of anaemia. Hematology 2000;5(1):77‐83. - PubMed
Jakobsen 2013 {published data only}
-
- Jakobsen D, Wiesenthal M, Hartmann F, Dignass A, Weber‐Mangal S, Stein J. Safety and efficacy of bolus administered ferric carboxymaltose (500 mg) in the treatment of iron deficiency anaemia in IBD patients. Journal of Crohn's and Colitis 2013;7(Suppl 1):S162.
Kanakaraddi 1973 {published data only}
-
- Kanakaraddi VP, Hoskatti CG, Nadig VS, Patil CK, Maiya M. Comparative therapeutic study of T.D.I. and I.M. injections of iron dextran complex in anaemia. Journal of Association of Physicians of India 1973;21(10):849‐53. - PubMed
Kang 2006 {published data only}
-
- Kang SB, Kim SC, Kim YT. Phase IV randomized study on safety and usefulness of parenteral iron (iron sucrose, venoferrum C) in the management of preoperative anemia in patients with menorrhagia: preliminary data. XVIII FIGO World Congress of Gynecology and Obstetrics 2006;2:89.
Kim 2007 {published data only}
-
- Kim YT, Kim SW, Yoon BS, Cho HJ, Nahm EJ, Kim SH, et al. Effect of intravenously administered iron sucrose on the prevention of anemia in the cervical cancer patients treated with concurrent chemoradiotherapy. Gynecologic Oncology 2007;105(1):199‐204. - PubMed
Kim 2009 {published data only}
-
- Kim YH, Chung HH, Kang SB, Kim SC, Kim YT. Safety and usefulness of intravenous iron sucrose in the management of preoperative anemia in patients with menorrhagia: a phase IV, open‐label, prospective, randomized study. Acta Haematologica 2009;121(1):37‐41. - PubMed
Kulnigg 2008 {published data only}
-
- Kulnigg S, Rumyantsev V, Stoinov S, Simanenkov V, Levchenko E, Karnafel W, et al. A novel intravenous iron formulation for treatment of anemia in IBD: the Ferinject randomized, controlled trial. Gastroenterology 2007;132(4 Suppl 2):A501‐2. - PubMed
-
- Kulnigg S, Rumyantsev V, Stoinov S, Simanenkov V, Levchenko E, Karnafel W, et al. A novel intravenous iron formulation for treatment of anemia in IBD: the Ferinject randomized, controlled trial. Zeitschrift fur Gastroenterologie 2007;44(A2):645‐6.
-
- Kulnigg S, Stoinov S, Simanenkov V, Dudar LV, Karnafel W, Garcia LC, et al. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. American Journal of Gastroenterology 2008;103(5):1182‐92. - PubMed
Langstaff 1993 {published data only}
-
- Langstaff RJ, Geisser P, Heil WG, Bowdler JM. Treatment of iron‐deficiency anaemia: a lower incidence of adverse effects with Ferrum Hausmann than ferrous sulphate. British Journal of Clinical Research 1993;4:191‐8.
Li 2005 {published data only}
-
- Li XX, Chen XL, Zhang MH, Wang YH, Da WM, Li JY. [A randomized controlled and multicenter clinical study of ferrous L‐threonate in treatment of iron deficiency anemia]. Zhonghua Nei Ke Aa Zhi [Chinese Journal of Internal Medicine] 2005;44(11):844‐7. - PubMed
Michalopoulou 2009 {published data only}
-
- Michalopoulou H, Vaitsis J, Massias S, Stamatis P. Effect of correction of anemia in severe congestive heart failure. Critical Care 2009;13:S168.
Mimura 2008 {published data only}
-
- Mimura EC, Bregano JW, Dichi JB, Gregorio EP, Dichi I. Comparison of ferrous sulfate and ferrous glycinate chelate for the treatment of iron deficiency anemia in gastrectomized patients. Nutrition 2008;24(7‐8):663‐8. - PubMed
NCT00199277 {published data only}
-
- Marti PP. Iron therapy in colo‐rectal neoplasm and iron deficiency anemia: intravenous iron sucrose versus oral ferrous sulphate. http://ClinicalTrials.gov/show/NCT00199277 2005:Accessed November 2014.
NCT00236951 {published data only}
-
- Trokars M. Intravenous (IV) iron vs. no iron as the treatment of anemia in cancer patients undergoing chemotherapy and erythropoietin therapy. http://ClinicalTrials.gov/show/NCT00236951 2010:Accessed November 2014.
NCT00482716 {published data only}
-
- Agrawal SG. Epoetin alfa or epoetin beta with or without iron infusion in treating anemia in patients with cancer. http://ClinicalTrials.gov/show/NCT00482716 2009:Accessed November 2014.
NCT00704028 {published data only}
-
- Tokars M. Safety and tolerability of ferric carboxymaltose (FCM) versus iron dextran in treating iron deficiency anemia. http://ClinicalTrials.gov/show/NCT00704028 2011:Accessed November 2014.
NCT00706667 {published data only}
-
- Theusinger O. Intravenous ferric carboxymaltose (Ferinject®) with or without erythropoietin in patients undergoing orthopaedic surgery. http://ClinicalTrials.gov/show/NCT00706667 2013:Accessed November 2014.
NCT00810030 {published data only}
-
- Gasche C. Ferinject for correction of anaemia in IBD patients, FER‐IBD‐COR. http://ClinicalTrials.gov/show/NCT00810030 2012:Accessed November 2014.
NCT00978575 {published data only}
-
- Vilstrup H. Iron substitution after upper gastro‐intestinal bleeding. http://ClinicalTrials.gov/show/NCT00978575 2013:Accessed November 2014.
NCT00982007 {published data only}
-
- Tokars M. Efficacy and safety of intravenous ferric carboxymaltose (FCM) in patients with iron deficiency anemia (IDA). http://ClinicalTrials.gov/show/NCT00982007 2013:Accessed November 2014.
NCT01017614 {published data only}
-
- Pharmacosmos A/S. Iron oligosaccharide in inflammatory bowel disease subjects with iron deficiency anaemia. http://ClinicalTrials.gov/show/NCT01017614 2012:Accessed November 2014.
NCT01100879 {published data only}
-
- Eirini K, Cushway T. Ferric carboxymaltose for treatment of anaemia of cancer in subjects with multiple myeloma receiving chemotherapy. http://ClinicalTrials.gov/show/NCT01100879 2011:Accessed November 2014.
NCT01101399 {published data only}
-
- Karlsson T, McNamara M. Ferric carboxymaltose in subjects with functional iron deficiency undergoing chemotherapy. http://ClinicalTrials.gov/show/NCT01101399 2012:Accessed November 2014.
NCT01114139 {published data only}
-
- AMAG Pharmaceuticals, Inc. A trial of ferumoxytol for the treatment of iron deficiency anemia. http://ClinicalTrials.gov/show/NCT01114139 2013:Accessed November 2014.
NCT01114204 {published data only}
-
- AMAG Pharmaceuticals, Inc. A trial comparing ferumoxytol with iron sucrose for the treatment of iron deficiency anemia. http://ClinicalTrials.gov/show/NCT01114204 2013:Accessed November 2014.
NCT01145638 {published data only}
-
- Thomsen LL. A study of intravenous iron isomaltoside 1000 (Monoferâ®) as monotherapy (without erythropoiesis stimulating agents) in comparison with oral iron sulfate in subjects with non‐myeloid malignancies associated with chemotherapy induced anaemia (CIA). http://ClinicalTrials.gov/show/NCT01145638 . Accessed November 2014.
NCT01160198 {published data only}
-
- GSK Clinical Trials. A study to demonstrate the efficacy and tolerability of ferrous bisglycinate chelate in iron deficiency anaemia and to compare these with those of ferrous ascorbate. http://ClinicalTrials.gov/show/NCT01160198 2012:Accessed November 2014.
NCT01180894 {published data only}
-
- Pieracci FM, Moore EE. IV iron for the anemia of traumatic critical illness. http://ClinicalTrials.gov/show/NCT01180894 2012:Accessed November 2014.
NCT01307007 {published data only}
-
- Tocars M. Hypophosphatemia with intravenous ferric carboxymaltose versus iron dextran in women with iron deficiency secondary to heavy uterine bleeding. http://ClinicalTrials.gov/show/NCT01307007 2013:Accessed November 2014.
NCT01309659 {published data only}
-
- Price E, Schrier S, Artiz A. Trial to evaluate the efficacy of intravenous iron in older adults with unexplained anemia. http://ClinicalTrials.gov/show/NCT01309659 2012:Accessed November 2014.
NCT01340872 {published data only}
-
- Harper C, Emery L. Safety and efficacy study of oral ferric iron to treat iron deficiency anaemia in quiescent ulcerative colitis (AEGIS‐1). http://ClinicalTrials.gov/show/NCT01340872 2011:Accessed November 2014.
NCT01352221 {published data only}
-
- Harper C, Emery L. Safety and efficacy study of oral ferric iron to treat iron deficiency anaemia in quiescent Crohn's disease (AEGIS‐2). http://ClinicalTrials.gov/show/NCT01352221 2011:Accessed November 2014.
NCT01425463 {published data only}
-
- UCB, Inc. A double‐blind, double‐dummy, parallel, active‐controlled, randomized and multi‐center trial to investigate efficacy and safety in subjects with iron deficiency anemia for ferrous (II) glycine sulphate complex versus polyferose capsules therapy. http://ClinicalTrials.gov/show/NCT01425463 2013:Accessed November 2014.
NCT01428843 {published data only}
-
- Savoye G, Colombel JF. Comparison of ferrisat vs placebo in anemia associated to inflammatory bowel disease during anti‐TNF therapy. http://ClinicalTrials.gov/show/NCT01428843 2012:Accessed November 2014.
NCT01690585 {published data only}
-
- Blot J. Efficacy of parenteral iron supplementation after gastrointestinal bleeding in subjects over 65. http://ClinicalTrials.gov/show/NCT01690585 2013:Accessed November 2014.
NCT01701310 {published data only}
-
- Keeler B, Ahceson AG. IVICA: intravenous iron in colorectal cancer associated anaemia. http://ClinicalTrials.gov/show/NCT01701310 2013:Accessed November 2014.
NCT01725789 {published data only}
-
- Kim YW. Ferinject® assessment in gastrectomy patients with acute isovolemic anemia (FAIRY). http://ClinicalTrials.gov/show/NCT01725789 2013:Accessed November 2014.
NCT01733979 {published data only}
-
- Chae SW. Efficacy and safety of heme‐iron polypeptide on improvement of anemia. http://ClinicalTrials.gov/show/NCT01733979 2012:Accessed November 2014.
NCT01837082 {published data only}
-
- Karakas M. Iron in congestive heart failure. http://ClinicalTrials.gov/show/NCT01837082 2013:Accessed November 2014.
NCT01857011 {published data only}
-
- Montano‐Pedroso JC. Iron supplementation for acute anemia after postbariatric abdominoplasty. http://ClinicalTrials.gov/show/NCT01857011 2013:Accessed November 2014.
NCT01927328 {published data only}
-
- Keeler B, Acheson AG. Iron replacement in oesophagogastric neoplasia. http://ClinicalTrials.gov/show/NCT01927328 2013:Accessed November 2014.
NCT01950247 {published data only}
-
- Koch T, Trokars M. Trial to evaluate the utility of serum hepcidin levels to predict response to oral or iv iron and to compare safety, effect on quality of life, and resource utilization of injectafer vs. intravenous standard of care for the treatment of iron deficiency anemia (IDA) in an infusion center setting. http://ClinicalTrials.gov/show/NCT01950247 2013:Accessed November 2014.
Okonko 2008 {published data only}
-
- Okonko D, Greszlo A, Witkowski T, Mandal A, Slater R, Roughton M, et al. Effect of intravenous iron sucrose on exercise tolerance in anaemic and nonanaemic patients with symptomatic chronic heart failure and iron deficiency (ferric‐HF): a randomised, controlled, observer‐blinded trial. Heart 2007;93(Suppl 1):A41. - PubMed
-
- Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Foldes G, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and non‐anemic patients with symptomatic chronic heart failure and iron deficiency: a randomised, controlled, observer‐blinded trial (FERRIC‐HF). Circulation 2006;114(18 Suppl S):407. - PubMed
-
- Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC‐HF: a randomized, controlled, observer‐blinded trial. Journal of the American College of Cardiology 2008;51(2):103‐12. - PubMed
-
- Okonko DO, Grzeslo A, Witkowski T, Missouris CG, Banasiak W, Poole‐Wilson PA, et al. Effect of intravenous iron sucrose on exercise tolerance in anaemic and non‐anaemic iron deficient patients with chronic heart failure: a randomised, controlled, observer‐blinded trial (FERRIC‐HF). European Heart Journal 2006;27(Suppl 1):167.
Olijhoek 2001 {published data only}
-
- Olijhoek G, Megens JGN, Musto P, Nogarin L, Gassmann‐Mayer C, Vercammen E, et al. Role of oral versus IV iron supplementation in the erythropoietic response to rHuEPO: a randomized, placebo‐controlled trial. Transfusion 2001;41(7):957‐63. - PubMed
Oliver 2010 {published data only}
-
- Oliver A, Sierra P, Noe L, Monllau V, Ortiz JC. Efficacy of post‐operative intravenous iron in elective major urological surgery. Transfusion Alternatives in Transfusion Medicine 2010;11(Suppl S2):33.
Pedrazzoli 1988 {published data only}
-
- Pedrazzoli P, Scotti A, Farina D. Comparison trial of iron succinylprotein complex or iron gluconate complex in the treatment of iron deficiency anemia. Clinical Therapeutics 1988;10(4):414‐20. - PubMed
Pedrazzoli 2008 {published data only}
-
- Pedrazzoli P, Farris A, Prete S, Gaizo F, Ferrari D, Bianchessi C, et al. Randomized trial of intravenous iron supplementation in patients with chemotherapy‐related anemia without iron deficiency treated with darbepoetin alpha. Journal of Clinical Oncology 2008;26(10):1619‐25. - PubMed
-
- Siena S, Farris A, Prete S, Gaizo F, Ferrari D, Bianchessi C, et al. Randomized trial of intravenous iron supplementation in patients with chemotherapy‐related anemia without iron deficiency treated with darbepoetin alfa. Annals of Oncology 2007;18(Suppl 11):87‐8. - PubMed
Prasad 2009 {published data only}
-
- Prasad N, Rajamani V, Hullin D, Murray JM. Post‐operative anaemia in femoral neck fracture patients: does it need treatment? A single blinded prospective randomised controlled trial. Injury 2009;40(10):1073‐6. - PubMed
Prassler 1998 {published data only}
-
- Prassler R, Deppe H, Huchzermeyer H. Value of iron supplementation in gastrointestinal bleeding. Leber Magen Darm 1998;28(1):21‐3.
Roe 1968 {published data only}
-
- Roe PF. The effect of varying concentrations of intravenous iron dextran (Imferon) on the complication rate. East African Medical Journal 1968;45(11):713‐9. - PubMed
Schroder 2005 {published data only}
-
- Schroder O, Mickisch O, Seidler U, Weerth A, Dignass AU, Herfarth H, et al. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease: a randomized, controlled, open‐label, multicenter study. American Journal of Gastroenterology 2005;100(11):2503‐9. - PubMed
-
- Schroder O, Mickisch O, Seidler U, Weerth A, Dignass AU, Herfarth H, et al. A randomised multi‐centre study on the application of intravenous iron sucrose versus oral iron sulphate in the therapy of iron deficient anaemia in patients with chronic irritable bowel disease. Zeitschrift fur Gastroenterologie 2005;43(8):915.
Seid 2006 {published data only}
-
- Seid MH, Mangione A, Valaoras TG, Anthony LB, Barish CF. Safety profile of iron carboxymaltose, a new high dose intravenous iron in patients with iron deficiency anemia. Blood 2006;108(11 Part 2):8.
Velhal 1991 {published data only}
-
- Velhal GD, Bichile SK, Pai NP, Tilwe S. Effectivness of oral versus parenteral iron therapy study conducted at urban health centre. Indian Journal of Community Medicine 1991;16(4):157‐60.
References to ongoing studies
NCT01692418 {published data only}
-
- Richards T. Preoperative intravenous iron to treat anaemia in major surgery. http://ClinicalTrials.gov/show/NCT01692418 2012. - PMC - PubMed
Additional references
Albaramki 2012
Bailie 2012
-
- Bailie GR. Comparison of rates of reported adverse events associated with i.v. iron products in the United States. American Journal of Health‐System Pharmacy 2012;69(4):310‐20. - PubMed
Balarajan 2011
-
- Balarajan Y, Ramakrishnan U, Ozaltin E, Shankar AH, Subramanian SV. Anaemia in low‐income and middle‐income countries. Lancet 2011;378(9809):2123‐35. - PubMed
Baribeault 2011
-
- Baribeault D, Auerbach M. Iron replacement therapy in cancer‐related anemia. American Journal of Health‐System Pharmacy 2011;68(10 Suppl 1):S4‐14. - PubMed
Beattie 2009
-
- Beattie WS, Karkouti K, Wijeysundera DN, Tait G. Risk associated with preoperative anemia in noncardiac surgery: a single‐center cohort study. Anesthesiology 2009;110(3):574‐81. - PubMed
Danielson 2004
-
- Danielson BG. Structure, chemistry, and pharmacokinetics of intravenous iron agents. Journal of the American Society of Nephrology 2004;15(Suppl 2):S93‐8. - PubMed
Davis 2012
-
- Davis SL, Littlewood TJ. The investigation and treatment of secondary anaemia. Blood Reviews 2012;26(2):65‐71. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
DeMets 1987
-
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Dodd 2004
Dunne 2002
-
- Dunne JR, Malone D, Tracy JK, Gannon C, Napolitano LM. Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. Journal of Surgical Research 2002;102(2):237‐44. - PubMed
Egger 1997
Fireman 2004
-
- Fireman Z, Kopelman Y. The role of video capsule endoscopy in the evaluation of iron deficiency anaemia. Digestive and Liver Disease 2004;36(2):97‐102. - PubMed
Foubert 2006
-
- Foubert J. Cancer‐related anaemia and fatigue: assessment and treatment. Nursing Standard 2006;20(36):50‐7. - PubMed
Ghali 2009
-
- Ghali JK. Anemia and heart failure. Current Opinion in Cardiology 2009;24(2):172‐8. - PubMed
Goddard 2011
-
- Goddard AF, James MW, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. Gut 2011 May 11 [Epub ahead of print]. - PubMed
Goonewardene 2012
-
- Goonewardene M, Shehata M, Hamad A. Anaemia in pregnancy. Best Practice & Research. Clinical Obstetrics & Gynaecology 2012;26(1):3‐24. - PubMed
Gurusamy 2009
-
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. The British Journal of Surgery 2009;96(4):342‐9. - PubMed
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Colloboration, 2011. www.cochrane‐handbook.org.
Higgins 2011a
-
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hyder 2002
-
- Hyder SM, Persson LA, Chowdhury AM, Ekstrom EC. Do side‐effects reduce compliance to iron supplementation? A study of daily‐ and weekly‐dose regimens in pregnancy. Journal of Health, Population, and Nutrition 2002;20(2):175‐9. - PubMed
ICH‐GCP 1996
-
- International Conference on Harmonisation of Technical Requiements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulation & ICH Guidelines. Media: Parexel Barnett, 1996.
Jain 2012
-
- Jain N, Jain VM. Prevalence of anemia in school children. Medical Practice and Review 2012;3(1):1‐4.
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Lee 2012
-
- Lee TW, Kolber MR, Fedorak RN, Zanten SV. Iron replacement therapy in inflammatory bowel disease patients with iron deficiency anemia: a systematic review and meta‐analysis. J Crohn's Colitis 2012;6(3):267‐75. - PubMed
Leichtle 2011
-
- Leichtle SW, Mouawad NJ, Lampman R, Singal B, Cleary RK. Does preoperative anemia adversely affect colon and rectal surgery outcomes?. Journal of the American College of Surgeons 2011;212(2):187‐94. - PubMed
Litton 2013
Liu 2012
-
- Liu K, Kaffes AJ. Iron deficiency anaemia: a review of diagnosis, investigation and management. European Journal of Gastroenterology & Hepatology 2012;24(2):109‐16. - PubMed
Lundh 2012
Macaskill 2001
-
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. - PubMed
McIntyre 2013
-
- McIntyre LA, Tinmouth A, Fergusson D. Intravenous iron therapy for treatment of anaemia. BMJ (Clinical Research ed) 2013;347:f5378. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
Musallam 2011
-
- Musallam KM, Tamim HM, Richards T, Spahn DR, Rosendaal FR, Habbal A, et al. Preoperative anemia and postoperative outcomes in noncardiac surgery. Lancet 2011;378(9800):1396‐407. - PubMed
NCBI‐Hematocrit
-
- NCBI. Hematocrit. http://www.ncbi.nlm.nih.gov/mesh/68006400 (accessed 19 July 2011).
NCBI‐Hemoglobins
-
- NCBI. Hemoglobins. http://www.ncbi.nlm.nih.gov/mesh/68006454 (accessed 19 July 2011).
NCBI‐Iron
-
- NCBI. Iron. http://www.ncbi.nlm.nih.gov/mesh/68007501 (accessed 19 July 2011).
Newell 1992
-
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. - PubMed
NLM Anemia
-
- U.S. National Library of Medicine. Anemia. http://www.ncbi.nlm.nih.gov/mesh/68000740 (accessed 19 July 2011).
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Reveiz 2011
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Santiago 2012
Scholz 1997
-
- Scholz BD, Gross R, Schultink W, Sastroamidjojo S. Anaemia is associated with reduced productivity of women workers even in less‐physically‐strenuous tasks. British Journal of Nutrition 1997;77(1):47‐57. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. Higgins JPT, Green S(editors). Cochrane Handbook for Systematic Reviewsof Interventions Version 5.1.0 [updated March 2011].The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Silverstein 2004
-
- Silverstein SB, Rodgers GM. Parenteral iron therapy options. American Journal of Hematology 2004;76(1):74‐8. - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Tierney 2007
von Haehling 2010
-
- Haehling S, Schefold JC, Hodoscek LM, Doehner W, Mannaa M, Anker SD, et al. Anaemia is an independent predictor of death in patients hospitalized for acute heart failure. Clinical Research in Cardiology 2010;99(2):107‐13. - PubMed
Waldmann 2004
-
- Waldmann A, Koschizke JW, Leitzmann C, Hahn A. Dietary iron intake and iron status of German female vegans: results of the German vegan study. Annals of Nutrition & Metabolism 2004;48(2):103‐8. - PubMed
Weiss 2005
-
- Weiss G, Goodnough LT. Anemia of chronic disease. The New England Journal of Medicine 2005;352(10):1011‐23. - PubMed
WHO 2001
-
- World Health Organization, United Nations University, UNICEF. Iron deficiency anaemia. Assessment, prevention, and control. A guide for managers. http://www.who.int/reproductivehealth/publications/maternal_perinatal_he... 2001:WHO/NHD/01.3 (accessed 19 August 2011).
WHO 2008
-
- World Health Organization. Worldwide prevalence of anaemia 1993‐2005. http://www.who.int/vmnis/publications/anaemia_prevalence/en/index.html. (accessed 19 August 2011).
Wood 2008
Yadav 2011
-
- Yadav D, Chandra J. Iron deficiency: beyond anemia. Indian Journal of Pediatrics 2011;78(1):65‐72. - PubMed
Zeng 2009
-
- Zeng X, Wu T. Iron supplementation for iron deficiency anemia in children. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006465] - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

