Systematic evaluation of antibody-mediated siRNA delivery using an industrial platform of THIOMAB-siRNA conjugates
- PMID: 25550431
- PMCID: PMC4333408
- DOI: 10.1093/nar/gku1362
Systematic evaluation of antibody-mediated siRNA delivery using an industrial platform of THIOMAB-siRNA conjugates
Abstract
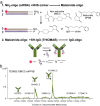
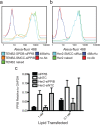
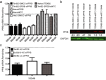
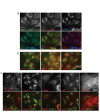
Delivery of siRNA is a key hurdle to realizing the therapeutic promise of RNAi. By targeting internalizing cell surface antigens, antibody-siRNA complexes provide a possible solution. However, initial reports of antibody-siRNA complexes relied on non-specific charged interactions and have not been broadly applicable. To assess and improve this delivery method, we built on an industrial platform of therapeutic antibodies called THIOMABs, engineered to enable precise covalent coupling of siRNAs. We report that such coupling generates monomeric antibody-siRNA conjugates (ARCs) that retain antibody and siRNA activities. To broadly assess this technology, we generated a battery of THIOMABs against seven targets that use multiple internalization routes, enabling systematic manipulation of multiple parameters that impact delivery. We identify ARCs that induce targeted silencing in vitro and extend tests to target prostate carcinoma cells following systemic administration in mouse models. However, optimal silencing was restricted to specific conditions and only observed using a subset of ARCs. Trafficking studies point to ARC entrapment in endocytic compartments as a limiting factor, independent of the route of antigen internalization. Our broad characterization of multiple parameters using therapeutic-grade conjugate technology provides a thorough assessment of this delivery technology, highlighting both examples of success as well as remaining challenges.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. - PubMed
-
- Tabernero J., Shapiro G.I., LoRusso P.M., Cervantes A., Schwartz G.K., Weiss G.J., Paz-Ares L., Cho D.C., Infante J.R., Alsina M., et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406–417. - PubMed
-
- Matsumura Y., Oda T., Maeda H. General mechanism of intratumor accumulation of macromolecules: advantage of macromolecular therapeutics. Cancer Chemother. 1987;14:821–829. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

