Increased Akt signaling in the mosquito fat body increases adult survivorship
- PMID: 25550465
- PMCID: PMC4396613
- DOI: 10.1096/fj.14-261479
Increased Akt signaling in the mosquito fat body increases adult survivorship
Abstract
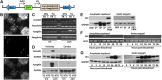
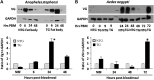
Akt signaling regulates diverse physiologies in a wide range of organisms. We examine the impact of increased Akt signaling in the fat body of 2 mosquito species, the Asian malaria mosquito Anopheles stephensi and the yellow fever mosquito Aedes aegypti. Overexpression of a myristoylated and active form of A. stephensi and Ae. aegypti Akt in the fat body of transgenic mosquitoes led to activation of the downstream signaling molecules forkhead box O (FOXO) and p70 S6 kinase in a tissue and blood meal-specific manner. In both species, increased Akt signaling in the fat body after blood feeding significantly increased adult survivorship relative to nontransgenic sibling controls. In A. stephensi, survivorship was increased by 15% to 45%, while in Ae. aegypti, it increased 14% to 47%. Transgenic mosquitoes fed only sugar, and thus not expressing active Akt, had no significant difference in survivorship relative to nontransgenic siblings. Expression of active Akt also increased expression of fat body vitellogenin, but the number of viable eggs did not differ significantly between transgenic and nontransgenic controls. This work demonstrates a novel mechanism of enhanced survivorship through increased Akt signaling in the fat bodies of multiple mosquito genera and provides new tools to unlock the molecular underpinnings of aging in eukaryotic organisms.
Keywords: Aedes aegypti; Anopheles stephensi; aging; insulin signaling; vitellogenin.
© FASEB.
Figures


References
-
- Antonova Y., Arik A., Moore W., Riehle M., Brown M. (2012) Insulin-like peptides: structure, signaling, and function. In Insect Endocrinology (Gilbert L., ed.), pp. 63–92, Elsevier, New York
-
- Toivonen J. M., Partridge L. (2009) Endocrine regulation of aging and reproduction in Drosophila. Mol. Cell. Endocrinol. 299, 39–50 - PubMed
-
- Mukhopadhyay A., Tissenbaum H. A. (2007) Reproduction and longevity: secrets revealed by C. elegans. Trends Cell Biol. 17, 65–71 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

