Recent advances in the electrochemical construction of heterocycles
- PMID: 25550752
- PMCID: PMC4273298
- DOI: 10.3762/bjoc.10.303
Recent advances in the electrochemical construction of heterocycles
Abstract
Due to the fact that the major portion of pharmaceuticals and agrochemicals contains heterocyclic units and since the overall number of commercially used heterocyclic compounds is steadily growing, heterocyclic chemistry remains in the focus of the synthetic community. Enormous efforts have been made in the last decades in order to render the production of such compounds more selective and efficient. However, most of the conventional methods for the construction of heterocyclic cores still involve the use of strong acids or bases, the operation at elevated temperatures and/or the use of expensive catalysts and reagents. In this regard, electrosynthesis can provide a milder and more environmentally benign alternative. In fact, numerous examples for the electrochemical construction of heterocycles have been reported in recent years. These cases demonstrate that ring formation can be achieved efficiently under ambient conditions without the use of additional reagents. In order to account for the recent developments in this field, a selection of representative reactions is presented and discussed in this review.
Keywords: anodic cyclization; electrosynthesis; heterocycle; olefin coupling; organic electrochemistry; radical cyclization.
Figures



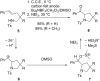




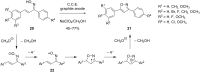
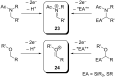




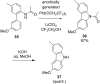
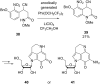

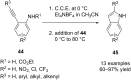

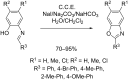










References
-
- Li J J, editor. Heterocyclic Chemistry in Drug Discovery. Hoboken: John Wiley & Sons; 2013.
-
- Lamberth C, Dinges J, editors. Bioactive Heterocyclic Compound Classes: Agrochemicals. Weinheim: Wiley-VCH; 2012.
-
- Alvarez-Builla J, Vaquero J J, Barluenga J, editors. Modern Heterocyclic Chemistry. Weinheim: Wiley-VCH; 2011.
-
- Joule J A, Mills K. Heterocyclic Chemistry. Hoboken: Wiley-Blackwell; 2010.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
