The efficacy and safety of certolizumab pegol (CZP) in the treatment of active rheumatoid arthritis (RA): a meta-analysis from nine randomized controlled trials
- PMID: 25550895
- PMCID: PMC4276153
The efficacy and safety of certolizumab pegol (CZP) in the treatment of active rheumatoid arthritis (RA): a meta-analysis from nine randomized controlled trials
Abstract
Objective: Certolizumab pegol (CZP) is a novel anti-TNF agent that is used for patients with moderate to severe active rheumatoid arthritis (RA). However, the efficacy of CZP in RA remains controversial. Thus, we performed this meta-analysis to assess the efficacy and safety of CZP in the treatment of RA patients.
Methods: Eligible studies were randomized controlled trials (RCTs) that evaluated the efficacy and safe of CZP in the patients with active RA. The primary outcome was American College of Rheumatology 20% (ACR20), and secondary outcome were ACR50, ACR70, disease activity, patient-reported outcomes (PROs), and adverse events. A fixed-effect model or random-effect model was used to pool the estimates, depending on the absence or presence of heterogeneity among the included studies.
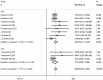
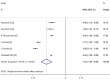
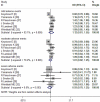
Results: Nine RCTs with a total of 5228 patients were included in this meta-analysis, and all of the patients were administered CZP or placebo. The pooled results showed that CZP significantly improved the ACR20, ACR50, ACR70 response rates, and physical function. CZP was associated with a statistically significant reduction in Disease Activity Score in 28 joints-Erythrocyte sedimentation rate, arthritis pain, and fatigue. Patients who received CZP treatment did not have a higher incidence of treatment-related adverse events, no matter in any intensity.
Conclusions: CZP 200 or 400mg in the treatment of active RA significantly reduced the RA signs and symptoms, and improved physical function as compared with the placebo. More large-scale RCTs are needed to evaluate the long-term efficacy and safety of CZP in the treatment of active RA.
Keywords: Certolizumab pegol; meta-analysis; rheumatoid arthritis.
Figures







References
-
- Maini RN, Brennan FM, Williams R, Chu CQ, Cope AP, Gibbons D, Elliott M, Feldmann M. TNF-alpha in rheumatoid arthritis and prospects of anti-TNF therapy. Clin Exp Rheumatol. 1993;11:S173–5. - PubMed
-
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, Teoh LA, Fischkoff SA, Chartash EK. Adalimumab, a fully human anti-tumor necrosis factor-monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. - PubMed
-
- Maini RN, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, St Clair EW, Keenan GF, van der Heijde D, Marsters PA, Lipsky PE Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004;50:1051–65. - PubMed
-
- Genovese MC, Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, Wasko MC, Moreland LW, Weaver AL, Markenson J, Cannon GW, Spencer-Green G, Finck BK. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum. 2002;46:1443–50. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
