Hybrid spin-crossover nanostructures
- PMID: 25551051
- PMCID: PMC4273211
- DOI: 10.3762/bjnano.5.232
Hybrid spin-crossover nanostructures
Abstract
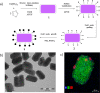
This review reports on the recent progress in the synthesis, modelling and application of hybrid spin-crossover materials, including core-shell nanoparticles and multilayer thin films or nanopatterns. These systems combine, often in synergy, different physical properties (optical, magnetic, mechanical and electrical) of their constituents with the switching properties of spin-crossover complexes, providing access to materials with unprecedented capabilities.
Keywords: core–shell particle; multifunctionality; nanomaterials; spin-crossover.
Figures









References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
