Classification, clinical features, and genetics of neural tube defects
- PMID: 25551113
- PMCID: PMC4362100
Classification, clinical features, and genetics of neural tube defects
Abstract
Neural tube defects (NTDs) constitute a major health burden (0.5-2/1000 pregnancies worldwide), and remain a preventable cause of still birth, neonatal, and infant death, or significant lifelong handicaps. The malformations result from failure of the neural folds to fuse in the midline, and form the neural tube between the third and the fourth week of embryonic development. This review article discusses their classification, clinical features, and genetics. Most NTDs are sporadic and both genetic, and non-genetic environmental factors are involved in its etiology. Consanguinity was suggested to contribute to the high incidence of NTDs in several countries, including Saudi Arabia. Syndromes, often associated with chromosomal anomalies, account for <10% of all NTDs; but a higher proportion (20%) has been documented in Saudi Arabia. Genetic predisposition constitutes the major underlying risk factor, with a strong implication of genes that regulate folate one-carbon metabolism and planar cell polarity.
Figures
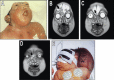




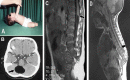

References
-
- Hamamy H. Epidemiological profile of neural tube defects in Arab countries. Middle East Journal of Medical Genetics. 2014;3:1–10.
-
- Bassuk AG, Kibar Z. Genetic basis of neural tube defects. Semin Pediatr Neurol. 2009;16:101–110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
