Midkine-deficiency delays chondrogenesis during the early phase of fracture healing in mice
- PMID: 25551381
- PMCID: PMC4281158
- DOI: 10.1371/journal.pone.0116282
Midkine-deficiency delays chondrogenesis during the early phase of fracture healing in mice
Abstract
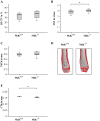
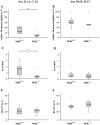
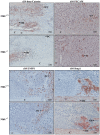
The growth and differentiation factor midkine (Mdk) plays an important role in bone development and remodeling. Mdk-deficient mice display a high bone mass phenotype when aged 12 and 18 months. Furthermore, Mdk has been identified as a negative regulator of mechanically induced bone formation and it induces pro-chondrogenic, pro-angiogenic and pro-inflammatory effects. Together with the finding that Mdk is expressed in chondrocytes during fracture healing, we hypothesized that Mdk could play a complex role in endochondral ossification during the bone healing process. Femoral osteotomies stabilized using an external fixator were created in wildtype and Mdk-deficient mice. Fracture healing was evaluated 4, 10, 21 and 28 days after surgery using 3-point-bending, micro-computed tomography, histology and immunohistology. We demonstrated that Mdk-deficient mice displayed delayed chondrogenesis during the early phase of fracture healing as well as significantly decreased flexural rigidity and moment of inertia of the fracture callus 21 days after fracture. Mdk-deficiency diminished beta-catenin expression in chondrocytes and delayed presence of macrophages during early fracture healing. We also investigated the impact of Mdk knockdown using siRNA on ATDC5 chondroprogenitor cells in vitro. Knockdown of Mdk expression resulted in a decrease of beta-catenin and chondrogenic differentiation-related matrix proteins, suggesting that delayed chondrogenesis during fracture healing in Mdk-deficient mice may be due to a cell-autonomous mechanism involving reduced beta-catenin signaling. Our results demonstrated that Mdk plays a crucial role in the early inflammation phase and during the development of cartilaginous callus in the fracture healing process.
Conflict of interest statement
Figures



Similar articles
-
PTH 1-34 Ameliorates the Osteopenia and Delayed Healing of Stabilized Tibia Fracture in Mice with Achondroplasia Resulting from Gain-Of-Function Mutation of FGFR3.Int J Biol Sci. 2017 Sep 21;13(10):1254-1265. doi: 10.7150/ijbs.21258. eCollection 2017. Int J Biol Sci. 2017. PMID: 29104492 Free PMC article.
-
Influence of Menopause on Inflammatory Cytokines during Murine and Human Bone Fracture Healing.Int J Mol Sci. 2018 Jul 16;19(7):2070. doi: 10.3390/ijms19072070. Int J Mol Sci. 2018. PMID: 30013010 Free PMC article.
-
Antagonizing midkine accelerates fracture healing in mice by enhanced bone formation in the fracture callus.Br J Pharmacol. 2016 Jul;173(14):2237-49. doi: 10.1111/bph.13503. Epub 2016 May 29. Br J Pharmacol. 2016. PMID: 27111560 Free PMC article.
-
Non-steroidal anti-inflammatory drugs, cyclooxygenase-2 and the bone healing process.Basic Clin Pharmacol Toxicol. 2008 Jan;102(1):10-4. doi: 10.1111/j.1742-7843.2007.00149.x. Epub 2007 Oct 31. Basic Clin Pharmacol Toxicol. 2008. PMID: 17973900 Review.
-
[Effect of BMP signaling in cartilage and bone formation].Clin Calcium. 2006 Apr;16(4):670- 74. Clin Calcium. 2006. PMID: 16582520 Review. Japanese.
Cited by
-
Inhibition of Midkine Augments Osteoporotic Fracture Healing.PLoS One. 2016 Jul 13;11(7):e0159278. doi: 10.1371/journal.pone.0159278. eCollection 2016. PLoS One. 2016. PMID: 27410432 Free PMC article.
-
The Role of the Intestinal Microbiome in Chronic Psychosocial Stress-Induced Pathologies in Male Mice.Front Behav Neurosci. 2018 Oct 26;12:252. doi: 10.3389/fnbeh.2018.00252. eCollection 2018. Front Behav Neurosci. 2018. PMID: 30464743 Free PMC article.
-
Hypochlorhydria-induced calcium malabsorption does not affect fracture healing but increases post-traumatic bone loss in the intact skeleton.J Orthop Res. 2016 Nov;34(11):1914-1921. doi: 10.1002/jor.23221. Epub 2016 Mar 14. J Orthop Res. 2016. PMID: 26945509 Free PMC article.
-
Acute kidney injury risk in orthopaedic trauma patients pre and post surgery using a biomarker algorithm and clinical risk score.Sci Rep. 2020 Nov 17;10(1):20005. doi: 10.1038/s41598-020-76929-y. Sci Rep. 2020. PMID: 33203963 Free PMC article.
-
Metabolic reprogramming of arachidonic acid in clear cell renal carcinoma promotes an immunosuppressive microenvironment by activating MDK signaling pathway.Clin Exp Med. 2025 Aug 13;25(1):291. doi: 10.1007/s10238-025-01807-8. Clin Exp Med. 2025. PMID: 40802073 Free PMC article.
References
-
- Ohta S, Muramatsu H, Senda T, Zou K, Iwata H, et al. (1999) Midkine is expressed during repair of bone fracture and promotes chondrogenesis. J Bone Miner Res 14:1132–1144. - PubMed
-
- Neunaber C, Catala-Lehnen P, Beil FT, Marshall RP, Kanbach V, et al. (2010) Increased trabecular bone formation in mice lacking the growth factor midkine. J Bone Miner Res 25:1724–1735. - PubMed
-
- Liedert A, Mattausch L, Rontgen V, Blakytny R, Vogele D, et al. (2011) Midkine-deficiency increases the anabolic response of cortical bone to mechanical loading. Bone 48:945–951. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

