Tau reduction diminishes spatial learning and memory deficits after mild repetitive traumatic brain injury in mice
- PMID: 25551452
- PMCID: PMC4281043
- DOI: 10.1371/journal.pone.0115765
Tau reduction diminishes spatial learning and memory deficits after mild repetitive traumatic brain injury in mice
Abstract
Objective: Because reduction of the microtubule-associated protein Tau has beneficial effects in mouse models of Alzheimer's disease and epilepsy, we wanted to determine whether this strategy can also improve the outcome of mild traumatic brain injury (TBI).
Methods: We adapted a mild frontal impact model of TBI for wildtype C57Bl/6J mice and characterized the behavioral deficits it causes in these animals. The Barnes maze, Y maze, contextual and cued fear conditioning, elevated plus maze, open field, balance beam, and forced swim test were used to assess different behavioral functions. Magnetic resonance imaging (MRI, 7 Tesla) and histological analysis of brain sections were used to look for neuropathological alterations. We also compared the functional effects of this TBI model and of controlled cortical impact in mice with two, one or no Tau alleles.
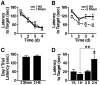
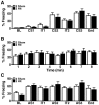
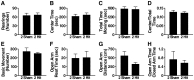
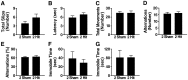
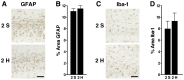
Results: Repeated (2-hit), but not single (1-hit), mild frontal impact impaired spatial learning and memory in wildtype mice as determined by testing of mice in the Barnes maze one month after the injury. Locomotor activity, anxiety, depression and fear related behaviors did not differ between injured and sham-injured mice. MRI imaging did not reveal focal injury or mass lesions shortly after the injury. Complete ablation or partial reduction of tau prevented deficits in spatial learning and memory after repeated mild frontal impact. Complete tau ablation also showed a trend towards protection after a single controlled cortical impact. Complete or partial reduction of tau also reduced the level of axonopathy in the corpus callosum after repeated mild frontal impact.
Interpretation: Tau promotes or enables the development of learning and memory deficits and of axonopathy after mild TBI, and tau reduction counteracts these adverse effects.
Conflict of interest statement
Figures

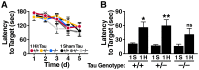

Similar articles
-
Traumatic injury to the immature frontal lobe: a new murine model of long-term motor impairment in the absence of psychosocial or cognitive deficits.Dev Neurosci. 2013;35(6):474-90. doi: 10.1159/000355874. Epub 2013 Nov 16. Dev Neurosci. 2013. PMID: 24247103 Free PMC article.
-
Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model.Ann Neurol. 2014 Feb;75(2):241-54. doi: 10.1002/ana.24064. Epub 2014 Feb 20. Ann Neurol. 2014. PMID: 24243523
-
Single mild traumatic brain injury results in transiently impaired spatial long-term memory and altered search strategies.Behav Brain Res. 2019 Jun 3;365:222-230. doi: 10.1016/j.bbr.2018.02.040. Epub 2018 Feb 27. Behav Brain Res. 2019. PMID: 29499284
-
CHIMERA repetitive mild traumatic brain injury induces chronic behavioural and neuropathological phenotypes in wild-type and APP/PS1 mice.Alzheimers Res Ther. 2019 Jan 12;11(1):6. doi: 10.1186/s13195-018-0461-0. Alzheimers Res Ther. 2019. PMID: 30636629 Free PMC article.
-
The effects of mild closed head injuries on tauopathy and cognitive deficits in rodents: Primary results in wild type and rTg4510 mice, and a systematic review.Exp Neurol. 2020 Apr;326:113180. doi: 10.1016/j.expneurol.2020.113180. Epub 2020 Jan 11. Exp Neurol. 2020. PMID: 31930992 Free PMC article.
Cited by
-
Neuropsychiatric Symptom Modeling in Male and Female C57BL/6J Mice after Experimental Traumatic Brain Injury.J Neurotrauma. 2017 Feb 15;34(4):890-905. doi: 10.1089/neu.2016.4508. Epub 2016 Jun 7. J Neurotrauma. 2017. PMID: 27149139 Free PMC article.
-
Humanized Tau Mice with Regionalized Amyloid Exhibit Behavioral Deficits but No Pathological Interaction.PLoS One. 2016 Apr 12;11(4):e0153724. doi: 10.1371/journal.pone.0153724. eCollection 2016. PLoS One. 2016. PMID: 27070146 Free PMC article.
-
Repeated Closed Head Injury in Mice Results in Sustained Motor and Memory Deficits and Chronic Cellular Changes.PLoS One. 2016 Jul 18;11(7):e0159442. doi: 10.1371/journal.pone.0159442. eCollection 2016. PLoS One. 2016. PMID: 27427961 Free PMC article.
-
Sex differences in cued fear responses and parvalbumin cell density in the hippocampus following repetitive concussive brain injuries in C57BL/6J mice.PLoS One. 2019 Sep 5;14(9):e0222153. doi: 10.1371/journal.pone.0222153. eCollection 2019. PLoS One. 2019. PMID: 31487322 Free PMC article.
-
Association between Y-Maze Acquisition Learning and Major Histocompatibility Complex Class II Polymorphisms in Mice.Biomed Res Int. 2018 Jul 19;2018:6381932. doi: 10.1155/2018/6381932. eCollection 2018. Biomed Res Int. 2018. PMID: 30112411 Free PMC article.
References
-
- DeKosky ST, Ikonomovic MD, Gandy S (2010) Traumatic brain injury: football, warfare, and long-term effects. Minn Med 93:46–47. - PubMed
-
- Pape TL, High WM Jr, St Andre J, Evans C, Smith B, et al. (2013) Diagnostic accuracy studies in mild traumatic brain injury: a systematic review and descriptive analysis of published evidence. PM R 5:856–881. - PubMed
-
- DeKosky ST, Ikonomovic MD, Gandy S (2010) Traumatic brain injury–football, warfare, and long-term effects. N Engl J Med 363:1293–1296. - PubMed
-
- Krainin BM, Forsten RD, Kotwal RS, Lutz RH, Guskiewicz KM (2011) Mild traumatic brain injury literature review and proposed changes to classification. J Spec Oper Med 11:38–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

