Multiple functions of the SNARE protein Snap29 in autophagy, endocytic, and exocytic trafficking during epithelial formation in Drosophila
- PMID: 25551675
- PMCID: PMC4502674
- DOI: 10.4161/15548627.2014.981913
Multiple functions of the SNARE protein Snap29 in autophagy, endocytic, and exocytic trafficking during epithelial formation in Drosophila
Abstract
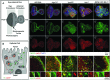
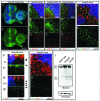
How autophagic degradation is linked to endosomal trafficking routes is little known. Here we screened a collection of uncharacterized Drosophila mutants affecting membrane transport to identify new genes that also have a role in autophagy. We isolated a loss of function mutant in Snap29 (Synaptosomal-associated protein 29 kDa), the gene encoding the Drosophila homolog of the human protein SNAP29 and have characterized its function in vivo. Snap29 contains 2 soluble NSF attachment protein receptor (SNARE) domains and a asparagine-proline-phenylalanine (NPF motif) at its N terminus and rescue experiments indicate that both SNARE domains are required for function, whereas the NPF motif is in part dispensable. We find that Snap29 interacts with SNARE proteins, localizes to multiple trafficking organelles, and is required for protein trafficking and for proper Golgi apparatus morphology. Developing tissue lacking Snap29 displays distinctive epithelial architecture defects and accumulates large amounts of autophagosomes, highlighting a major role of Snap29 in autophagy and secretion. Mutants for autophagy genes do not display epithelial architecture or secretion defects, suggesting that the these alterations of the Snap29 mutant are unlikely to be caused by the impairment of autophagy. In contrast, we find evidence of elevated levels of hop-Stat92E (hopscotch-signal transducer and activator of transcription protein at 92E) ligand, receptor, and associated signaling, which might underlie the epithelial defects. In summary, our findings support a role of Snap29 at key steps of membrane trafficking, and predict that signaling defects may contribute to the pathogenesis of cerebral dysgenesis, neuropathy, ichthyosis, and palmoplantar keratoderma (CEDNIK), a human congenital syndrome due to loss of Snap29.
Keywords: Atg, autophagy-related; CEDNIK, cerebral dysgenesis, neuropathy, ichthyosis, and palmoplantar keratoderma; CFP, cyan fluorescent protein; E(spl)mβ-HLH, enhancer of split mβ, helix-loop-helix; EM, electron microscopy; ESCRT, endosomal sorting complex required for transport; FE, follicular epithelium; GFP, green fluorescent protein; MENE, mutant eye no eclosion; MVB, multivesicular body; N, Notch; NECD, N extracellular domain; NPF, asparagine-proline-phenylalanine; Notch; SNARE; SNARE, soluble NSF attachment protein receptor; Snap29; Snap29, synaptosomal-associated protein 29 kDa; Socs36E, suppressor of cytokine signaling at 36E; Syb, Synaptobrevin; Syx, syntaxin; V-ATPase, vacuolar H+-ATPase; Vamp, vesicle-associated membrane protein; Vps25, vacuolar protein sorting 25; WT, wild type; autophagy; dome; dome, domeless; histone H3, His3; hop-Stat92E, hopscotch-signal transducer and activator of transcription protein at 92E; os, outstretched; ref(2)P, refractory to sigma P; trafficking; usnp.
Figures







References
-
- Vaccari T, Bilder D. At the crossroads of polarity, proliferation and apoptosis: the use of Drosophila to unravel the multifaceted role of endocytosis in tumor suppression. Mol Oncol 2009; 3:354-65; PMID:19560990; http://dx.doi.org/10.1016/j.molonc.2009.05.005 - DOI - PMC - PubMed
-
- Vaccari T, Bilder D. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell 2005; 9:687-98; PMID:16256743; http://dx.doi.org/10.1016/j.devcel.2005.09.019 - DOI - PubMed
-
- Woodfield SE, Graves HK, Hernandez JA, Bergmann A. De-regulation of JNK and JAKSTAT signaling in ESCRT-II mutant tissues cooperatively contributes to neoplastic tumorigenesis. PLoS One 2013; 8:e56021; PMID:23418496; http://dx.doi.org/10.1371/journal.pone.0056021 - DOI - PMC - PubMed
-
- Juhasz G, Erdi B, Sass M, Neufeld TP. Atg7-dependent autophagy promotes neuronal health, stress tolerance, and longevity but is dispensable for metamorphosis in Drosophila. Genes Dev 2007; 21:3061-6; PMID:18056421; http://dx.doi.org/10.1101/gad.1600707 - DOI - PMC - PubMed
-
- Takats S, Nagy P, Varga A, Pircs K, Karpati M, Varga K, Kovács AL, Hegedűs K, Juhász G. Autophagosomal Syntaxin17-dependent lysosomal degradation maintains neuronal function in Drosophila. J Cell Biol 2013; 201:531-9; PMID:23671310; http://dx.doi.org/10.1083/jcb.201211160 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
