Lipid rafts: integrated platforms for vascular organization offering therapeutic opportunities
- PMID: 25552244
- PMCID: PMC11113367
- DOI: 10.1007/s00018-014-1814-x
Lipid rafts: integrated platforms for vascular organization offering therapeutic opportunities
Abstract
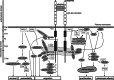
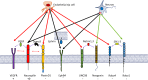
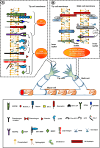
Research on the nanoscale membrane structures known as lipid rafts is relevant to the fields of cancer biology, inflammation and ischaemia. Lipid rafts recruit molecules critical to signalling and regulation of the invasion process in malignant cells, the leukocytes that provide immunity in inflammation and the endothelial cells that build blood and lymphatic vessels, as well as the patterning of neural networks. As angiogenesis is a common denominator, regulation of receptors and signalling molecules critical to angiogenesis is central to the design of new approaches aimed at reducing, promoting or normalizing the angiogenic process. The goal of this review is to highlight some of the key issues that indicate the involvement of endothelial cell lipid rafts at each step of so-called 'sprouting angiogenesis', from stimulation of the vascular endothelial growth factor to the choice of tip cells, activation of migratory and invasion pathways, recruitment of molecules that guide axons in vascular patterning and maturation of blood vessels. Finally, the review addresses opportunities for future studies to define how these lipid domains (and their constituents) may be manipulated to stimulate the so-called 'normalization' of vascular networks within tumors, and be identified as the main target, enabling the development of more efficient chemotherapeutics and cancer immunotherapies.
Figures

References
-
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. - PubMed
-
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. - PubMed
-
- Roura S, Gálvez-Montón C, Bayes-Genis A. The challenges for cardiac vascular precursor cell therapy: lessons from a very elusive precursor. J Vasc Res. 2013;50:304–323. - PubMed
-
- Gerhardt H, Betsholtz C. How do endothelial cells orientate? Experientia. 2005;94:3–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

