The structures of glycophorin C N-glycans, a putative component of the GPC receptor site for Plasmodium falciparum EBA-140 ligand
- PMID: 25552259
- PMCID: PMC4840387
- DOI: 10.1093/glycob/cwu188
The structures of glycophorin C N-glycans, a putative component of the GPC receptor site for Plasmodium falciparum EBA-140 ligand
Abstract
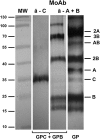
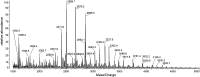
Glycophorins C and D are highly glycosylated integral sialoglycoproteins of human red blood cell membranes carrying the Gerbich blood group antigens. The O- and N-glycosidic chains of the major erythrocyte glycoprotein (Lisowska E. 2001, Antigenic properties of human glycophorins - an update. Adv Exp Med Biol, 491:155-169; Tomita M and Marchesi VT. 1975, Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci USA, 72:2964-2968.) are well characterized but the structure of GPC N-glycans has remained unknown. This problem became important since it was reported that GPC N-glycans play an essential role in the interaction with Plasmodium falciparum EBA-140 merozoite ligand. The elucidation of these structures seems essential for full characterization of the GPC binding site for the EBA-140 ligand. We have employed detailed structural analysis using sequential mass spectrometry to show that many GPC N-glycans contain H2 antigen structures and several contain polylactosamine structures capped with fucose. The results obtained indicate structural heterogeneity of the GPC N-glycans and show the existence of structural elements not found in glycophorin A N-glycans. Our results also open a possibility of new interpretation of the data concerning the binding of P. falciparum EBA-140 ligand to GPC. We hypothesize that preferable terminal fucosylation of N-glycosidic chains containing repeating lactosamine units of the GPC Gerbich variant could be an explanation for why the EBA-140 ligand does not react with GPC Gerbich and an indication that the EBA-140 interaction with GPC is distinctly dependent on the GPC N-glycan structure.
Keywords: EBA-140 ligand; Plasmodium; glycan; glycophorin; mass spectrometry.
© The Author 2014. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures




References
-
- Blanchard D, Dahr W, Hummel M, Latron F, Beyreuther K, Cartron JP. 1987. Glycophorins B and C from human erythrocyte membranes. Purification and sequence analysis. J Biol Chem. 262:5808–5811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

