Mitochondrial depolarization and asystole in the globally ischemic rabbit heart: coordinated response to interventions affecting energy balance
- PMID: 25552307
- PMCID: PMC4346765
- DOI: 10.1152/ajpheart.00257.2014
Mitochondrial depolarization and asystole in the globally ischemic rabbit heart: coordinated response to interventions affecting energy balance
Abstract
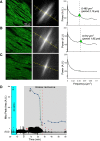
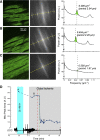
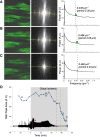
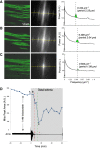
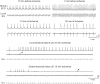
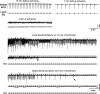
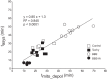
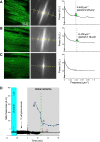
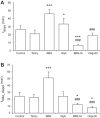
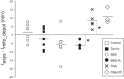
Mitochondrial membrane potential (ΔΨm) depolarization has been implicated in the loss of excitability (asystole) during global ischemia, which is relevant for the success of defibrillation and resuscitation after cardiac arrest. However, the relationship between ΔΨm depolarization and asystole during no-flow ischemia remains unknown. We applied spatial Fourier analysis to confocally recorded fluorescence emitted by ΔΨm-sensitive dye tetramethylrhodamine methyl ester. The time of ischemic ΔΨm depolarization (tmito_depol) was defined as the time of 50% decrease in the magnitude of spectral peaks reflecting ΔΨm. The time of asystole (tasys) was determined as the time when spontaneous and induced ventricular activity ceased to exist. Interventions included tachypacing (150 ms), myosin II ATPase inhibitor blebbistatin (heart immobilizer), and the combination of blebbistatin and the inhibitor of glycolysis iodoacetate. In the absence of blebbistatin, confocal images were obtained during brief perfusion with hyperkalemic solution and after the contraction failed between 7 and 15 min of ischemia. In control, tmito_depol and tasys were 24.4 ± 6.0 and 26.0 ± 5.0 min, respectively. Tachypacing did not significantly affect either parameter. Blebbistatin dramatically delayed tmito_depol and tasys (51.4 ± 8.6 and 45.7 ± 5.3 min, respectively; both P < 0.0001 vs. control). Iodoacetate combined with blebbistatin accelerated both events (tmito_depol, 12.7 ± 1.8 min; and tasys, 6.5 ± 1.1 min; both P < 0.03 vs. control). In all groups pooled together, tasys was strongly correlated with tmito_depol (R(2) = 0.845; P < 0.0001). These data may indicate a causal relationship between ΔΨm depolarization and asystole or a similar dependence of the two events on energy depletion during ischemia. Our results urge caution against the use of blebbistatin in studies addressing pathophysiology of myocardial ischemia.
Keywords: ATP-sensitive potassium channel; asystole; blebbistatin; mitochondrial depolarization; myocardial ischemia.
Copyright © 2015 the American Physiological Society.
Figures

Similar articles
-
Impact of levosimendan and ischaemia-reperfusion injury on myocardial subsarcolemmal mitochondrial respiratory chain, mitochondrial membrane potential, Ca2+ cycling and ATP synthesis.Eur J Cardiothorac Surg. 2016 Feb;49(2):e54-62; discussion e62. doi: 10.1093/ejcts/ezv397. Epub 2015 Nov 18. Eur J Cardiothorac Surg. 2016. PMID: 26586791
-
Mitochondrial instability during regional ischemia-reperfusion underlies arrhythmias in monolayers of cardiomyocytes.J Mol Cell Cardiol. 2015 Jan;78:90-9. doi: 10.1016/j.yjmcc.2014.09.024. Epub 2014 Sep 28. J Mol Cell Cardiol. 2015. PMID: 25268650 Free PMC article.
-
Undiminished mitochondrial function during stunning in rabbit heart at 28 degrees C.Cardiovasc Res. 1997 Jul;35(1):113-9. doi: 10.1016/s0008-6363(97)00104-1. Cardiovasc Res. 1997. PMID: 9302354
-
Detection of mitochondrial depolarization/recovery during ischaemia--reperfusion using spectral properties of confocally recorded TMRM fluorescence.J Physiol. 2013 Jun 1;591(11):2781-94. doi: 10.1113/jphysiol.2012.248153. Epub 2013 Mar 25. J Physiol. 2013. PMID: 23529126 Free PMC article.
-
Mitochondrial Bioenergetics During Ischemia and Reperfusion.Adv Exp Med Biol. 2017;982:141-167. doi: 10.1007/978-3-319-55330-6_8. Adv Exp Med Biol. 2017. PMID: 28551786 Review.
Cited by
-
Optical Mapping of Cardiac Electromechanics.Biophys J. 2016 Jul 26;111(2):269-270. doi: 10.1016/j.bpj.2016.04.052. Biophys J. 2016. PMID: 27463128 Free PMC article. No abstract available.
-
β-Adrenergic stimulation and rapid pacing mutually promote heterogeneous electrical failure and ventricular fibrillation in the globally ischemic heart.Am J Physiol Heart Circ Physiol. 2015 May 1;308(9):H1155-70. doi: 10.1152/ajpheart.00768.2014. Epub 2015 Feb 20. Am J Physiol Heart Circ Physiol. 2015. PMID: 25713306 Free PMC article.
-
Heated environment increases blood pressure drop and postural sway during initial orthostasis in healthy subjects.Eur J Appl Physiol. 2024 Nov;124(11):3365-3375. doi: 10.1007/s00421-024-05546-8. Epub 2024 Jun 27. Eur J Appl Physiol. 2024. PMID: 38935152
-
A technical review of optical mapping of intracellular calcium within myocardial tissue.Am J Physiol Heart Circ Physiol. 2016 Jun 1;310(11):H1388-401. doi: 10.1152/ajpheart.00665.2015. Epub 2016 Mar 25. Am J Physiol Heart Circ Physiol. 2016. PMID: 27016580 Free PMC article. Review.
-
Stop the beat to see the rhythm: excitation-contraction uncoupling in cardiac research.Am J Physiol Heart Circ Physiol. 2021 Dec 1;321(6):H1005-H1013. doi: 10.1152/ajpheart.00477.2021. Epub 2021 Oct 8. Am J Physiol Heart Circ Physiol. 2021. PMID: 34623183 Free PMC article. Review.
References
-
- Abi-Gerges N, Pointon A, Pullen GF, Morton MJ, Oldman KL, Armstrong D, Valentin JP, Pollard CE. Preservation of cardiomyocytes from the adult heart. J Mol Cell Cardiol 64: 108–119, 2013. - PubMed
-
- Baker LC, Wolk R, Choi BR, Watkins S, Plan P, Shah A, Salama G. Effects of mechanical uncouplers, diacetyl monoxime, and cytochalasin-D on the electrophysiology of perfused mouse hearts. Am J Physiol Heart Circ Physiol 287: H1771–H1779, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

