Radiation-induced lung injury and inflammation in mice: role of inducible nitric oxide synthase and surfactant protein D
- PMID: 25552309
- PMCID: PMC4349136
- DOI: 10.1093/toxsci/kfu255
Radiation-induced lung injury and inflammation in mice: role of inducible nitric oxide synthase and surfactant protein D
Abstract
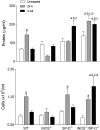
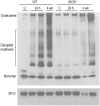
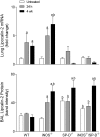
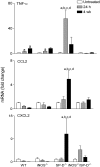
Reactive nitrogen species (RNS) generated after exposure to radiation have been implicated in lung injury. Surfactant protein D (SP-D) is a pulmonary collectin that suppresses inducible nitric oxide synthase (iNOS)-mediated RNS production. Herein, we analyzed the role of iNOS and SP-D in radiation-induced lung injury. Exposure of wild-type (WT) mice to γ-radiation (8 Gy) caused acute lung injury and inflammation, as measured by increases in bronchoalveolar lavage (BAL) protein and cell content at 24 h. Radiation also caused alterations in SP-D structure at 24 h and 4 weeks post exposure. These responses were blunted in iNOS(-/-) mice. Conversely, loss of iNOS had no effect on radiation-induced expression of phospho-H2A.X or tumor necrosis factor (TNF)-α. Additionally, at 24 h post radiation, cyclooxygenase expression and BAL lipocalin-2 levels were increased in iNOS(-/-) mice, and heme oxygenase (HO)-1(+) and Ym1(+) macrophages were evident. Loss of SP-D resulted in increased numbers of enlarged HO-1(+) macrophages in the lung following radiation, along with upregulation of TNF-α, CCL2, and CXCL2, whereas expression of phospho-H2A.X was diminished. To determine if RNS play a role in the altered sensitivity of SP-D(-/-) mice to radiation, iNOS(-/-)/SP-D(-/-) mice were used. Radiation-induced injury, oxidative stress, and tissue repair were generally similar in iNOS(-/-)/SP-D(-/-) and SP-D(-/-) mice. In contrast, TNF-α, CCL2, and CXCL2 expression was attenuated. These data indicate that although iNOS is involved in radiation-induced injury and altered SP-D structure, in the absence of SP-D, it functions to promote proinflammatory signaling. Thus, multiple inflammatory pathways contribute to the pathogenic response to radiation.
Keywords: iNOS; lung injury; radiation; reactive nitrogen species; surfactant protein D.
© The Author 2014. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Role of reactive nitrogen species generated via inducible nitric oxide synthase in vesicant-induced lung injury, inflammation and altered lung functioning.Toxicol Appl Pharmacol. 2012 May 15;261(1):22-30. doi: 10.1016/j.taap.2012.03.004. Epub 2012 Mar 14. Toxicol Appl Pharmacol. 2012. PMID: 22446026 Free PMC article.
-
Role of inducible nitric oxide synthase-derived nitric oxide in lipopolysaccharide plus interferon-gamma-induced pulmonary inflammation.Toxicol Appl Pharmacol. 2004 Feb 15;195(1):45-54. doi: 10.1016/j.taap.2003.10.005. Toxicol Appl Pharmacol. 2004. PMID: 14962504
-
Role of inducible nitric oxide synthase-derived nitric oxide in silica-induced pulmonary inflammation and fibrosis.J Toxicol Environ Health A. 2004 Jul 9;67(13):1001-26. doi: 10.1080/15287390490447296. J Toxicol Environ Health A. 2004. PMID: 15205031
-
Nitric oxide and peroxynitrite in ozone-induced lung injury.Adv Exp Med Biol. 2001;500:183-90. doi: 10.1007/978-1-4615-0667-6_24. Adv Exp Med Biol. 2001. PMID: 11764933 Review.
-
[New paradigm of host defense against intracellular pathogens by nitric oxide].Nihon Hansenbyo Gakkai Zasshi. 2009 Feb;78(1):41-7. doi: 10.5025/hansen.78.41. Nihon Hansenbyo Gakkai Zasshi. 2009. PMID: 19227148 Review. Japanese.
Cited by
-
Editor's Highlight: Role of Spleen-Derived Macrophages in Ozone-Induced Lung Inflammation and Injury.Toxicol Sci. 2017 Jan;155(1):182-195. doi: 10.1093/toxsci/kfw192. Epub 2016 Oct 5. Toxicol Sci. 2017. PMID: 27708193 Free PMC article.
-
Metabolomic Analysis of Radiation-Induced Lung Injury in Rats: The Potential Radioprotective Role of Taurine.Dose Response. 2019 Oct 30;17(4):1559325819883479. doi: 10.1177/1559325819883479. eCollection 2019 Oct-Dec. Dose Response. 2019. PMID: 31700502 Free PMC article.
-
A Potential Biomarker for Predicting the Risk of Radiation-Induced Fibrosis in the Lung.Radiat Res. 2018 Nov;190(5):513-525. doi: 10.1667/RR15122.1. Epub 2018 Aug 17. Radiat Res. 2018. PMID: 30117783 Free PMC article.
-
Promising role of filgrastim and α-tocopherol succinate in amelioration of gastrointestinal acute radiation syndrome (GI-ARS) in mice.Naunyn Schmiedebergs Arch Pharmacol. 2019 Dec;392(12):1537-1550. doi: 10.1007/s00210-019-01702-6. Epub 2019 Jul 27. Naunyn Schmiedebergs Arch Pharmacol. 2019. PMID: 31350581
-
Approaches to Evaluate Lung Inflammation in Translational Research.Vet Pathol. 2018 Jan;55(1):42-52. doi: 10.1177/0300985817726117. Epub 2017 Aug 16. Vet Pathol. 2018. PMID: 28812529 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

