Impaired limbic cortico-striatal structure and sustained visual attention in a rodent model of schizophrenia
- PMID: 25552430
- PMCID: PMC4368881
- DOI: 10.1093/ijnp/pyu010
Impaired limbic cortico-striatal structure and sustained visual attention in a rodent model of schizophrenia
Erratum in
-
Erratum.Int J Neuropsychopharmacol. 2016 Apr 27;19(10):pyw031. doi: 10.1093/ijnp/pyw031. Int J Neuropsychopharmacol. 2016. PMID: 27207904 Free PMC article. No abstract available.
Abstract
Background: N-methyl-d-aspartate receptor (NMDAR) dysfunction is thought to contribute to the pathophysiology of schizophrenia. Accordingly, NMDAR antagonists such as phencyclidine (PCP) are used widely in experimental animals to model cognitive impairment associated with this disorder. However, it is unclear whether PCP disrupts the structural integrity of brain areas relevant to the profile of cognitive impairment in schizophrenia.
Methods: Here we used high-resolution magnetic resonance imaging and voxel-based morphometry to investigate structural alterations associated with sub-chronic PCP treatment in rats.
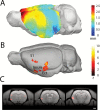
Results: Sub-chronic exposure of rats to PCP (5mg/kg twice daily for 7 days) impaired sustained visual attention on a 5-choice serial reaction time task, notably when the attentional load was increased. In contrast, sub-chronic PCP had no significant effect on the attentional filtering of a pre-pulse auditory stimulus in an acoustic startle paradigm. Voxel-based morphometry revealed significantly reduced grey matter density bilaterally in the hippocampus, anterior cingulate cortex, ventral striatum, and amygdala. PCP-treated rats also exhibited reduced cortical thickness in the insular cortex.
Conclusions: These findings demonstrate that sub-chronic NMDA receptor antagonism is sufficient to produce highly-localized morphological abnormalities in brain areas implicated in the pathogenesis of schizophrenia. Furthermore, PCP exposure resulted in dissociable impairments in attentional function.
Keywords: 5-CSRTT; PCP; VBM; anterior cingulate cortex; hippocampus.
© The Author 2014. Published by Oxford University Press on behalf of CINP.
Figures




References
-
- Aas M, Navari S, Gibbs A, Mondelli V, Fisher HL, Morgan C, Morgan K, MacCabe J, Reichenberg A, Zanelli J, Fearon P, Jones PB, Murray RM, Pariante CM, Dazzan P. (2012). Is there a link between childhood trauma, cognition, and amygdala and hippocampus volume in first-episode psychosis? Schizophr Res 137:73–79. - PubMed
-
- Abdul-Monim Z, Neill JC, Reynolds GP (2007). Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. J Psychopharmacol 21:198–205. - PubMed
-
- Abela AR, Dougherty SD, Fagen ED, Hill CJ, Chudasama Y. (2013). Inhibitory control deficits in rats with ventral hippocampal lesions. Cereb Cortex 23:1396–1409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

