A randomised controlled non-inferiority trial of primary care-based facilitated access to an alcohol reduction website (EFAR Spain): the study protocol
- PMID: 25552616
- PMCID: PMC4281557
- DOI: 10.1136/bmjopen-2014-007130
A randomised controlled non-inferiority trial of primary care-based facilitated access to an alcohol reduction website (EFAR Spain): the study protocol
Abstract
Introduction: Early identification (EI) and brief interventions (BIs) for risky drinkers are effective tools in primary care. Lack of time in daily practice has been identified as one of the main barriers to implementation of BI. There is growing evidence that facilitated access by primary healthcare professionals (PHCPs) to a web-based BI can be a time-saving alternative to standard face-to-face BIs, but there is as yet no evidence about the effectiveness of this approach relative to conventional BI. The main aim of this study is to test non-inferiority of facilitation to a web-based BI for risky drinkers delivered by PHCP against face-to-face BI.
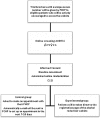
Method and analysis: A randomised controlled non-inferiority trial comparing both interventions will be performed in primary care health centres in Catalonia, Spain. Unselected adult patients attending participating centres will be given a leaflet inviting them to log on to a website to complete the Alcohol Use Disorders Identification Test (AUDIT-C) alcohol screening questionnaire. Participants with positive results will be requested online to complete a trial module including consent, baseline assessment and randomisation to either face-to-face BI by the practitioner or BI via the alcohol reduction website. Follow-up assessment of risky drinking will be undertaken online at 3 months and 1 year using the full AUDIT and D5-EQD5 scale. Proportions of risky drinkers in each group will be calculated and non-inferiority assessed against a specified margin of 10%. Assuming reduction of 30% of risky drinkers receiving standard intervention, 1000 patients will be required to give 90% power to reject the null hypothesis.
Ethics and dissemination: The protocol was approved by the Ethics Commmittee of IDIAP Jordi Gol i Gurina P14/028. The findings of the trial will be disseminated through peer-reviewed journals, national and international conference presentations.
Trial registration number: ClinicalTrials.gov NCT02082990.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
Similar articles
-
A randomised controlled non-inferiority trial of primary care-based facilitated access to an alcohol reduction website (EFAR-FVG): the study protocol.BMJ Open. 2013 Feb 12;3(2):e002304. doi: 10.1136/bmjopen-2012-002304. Print 2013. BMJ Open. 2013. PMID: 23408073 Free PMC article.
-
Randomised controlled non-inferiority trial of primary care-based facilitated access to an alcohol reduction website.BMJ Open. 2017 Nov 3;7(11):e014576. doi: 10.1136/bmjopen-2016-014576. BMJ Open. 2017. PMID: 29102982 Free PMC article. Clinical Trial.
-
A randomized controlled non-inferiority trial of primary care-based facilitated access to an alcohol reduction website (EFAR Spain).Internet Interv. 2021 Aug 20;26:100446. doi: 10.1016/j.invent.2021.100446. eCollection 2021 Dec. Internet Interv. 2021. PMID: 34522625 Free PMC article.
-
Screening and brief intervention for alcohol and other abuse.Adolesc Med State Art Rev. 2014 Apr;25(1):126-56. Adolesc Med State Art Rev. 2014. PMID: 25022191 Review.
-
Optimizing the delivery of interventions for harmful alcohol use in primary healthcare: an update.Curr Opin Psychiatry. 2018 Jul;31(4):324-332. doi: 10.1097/YCO.0000000000000435. Curr Opin Psychiatry. 2018. PMID: 29846264
Cited by
-
Community- and mHealth-based integrated management of diabetes in primary healthcare in Rwanda (D²Rwanda): the protocol of a mixed-methods study including a cluster randomised controlled trial.BMJ Open. 2019 Jul 24;9(7):e028427. doi: 10.1136/bmjopen-2018-028427. BMJ Open. 2019. PMID: 31345971 Free PMC article.
-
Implementing referral to an electronic alcohol brief advice website in primary healthcare: results from the ODHIN implementation trial.BMJ Open. 2016 Jun 16;6(6):e010271. doi: 10.1136/bmjopen-2015-010271. BMJ Open. 2016. PMID: 27311902 Free PMC article. Clinical Trial.
-
Clinician experiences of healthy lifestyle promotion and perceptions of digital interventions as complementary tools for lifestyle behavior change in primary care.BMC Fam Pract. 2018 Aug 21;19(1):139. doi: 10.1186/s12875-018-0829-z. BMC Fam Pract. 2018. PMID: 30131057 Free PMC article.
-
Download Your Doctor: Implementation of a Digitally Mediated Personal Physician Presence to Enhance Patient Engagement With a Health-Promoting Internet Application.JMIR Res Protoc. 2016 Mar 4;5(1):e36. doi: 10.2196/resprot.5232. JMIR Res Protoc. 2016. PMID: 26944482 Free PMC article.
-
Validation of the AUDIT-C in adults seeking help with their drinking online.Addict Sci Clin Pract. 2017 Jan 4;12(1):2. doi: 10.1186/s13722-016-0066-5. Addict Sci Clin Pract. 2017. PMID: 28049515 Free PMC article.
References
-
- Wolstenholme A, Drummond C, Deluca P, et al. Report on the mapping of European need and service provision for early diagnosis and treatment of alcohol use disorders. Deliverable 2.5, Work Package 6. The public health impact of individually directed brief interventions and treatment for alcohol use disorders in European countries. http://amphoraproject.net/w2box/data/Deliverables/AMPHORA_WP6_D2.5.pdf.
-
- Drummond C, Wolstenholme A, Deluca P et al. . Alcohol interventions and treatment in Europe. In: Anderson P, Braddick F, Reynolds J, Gual A, eds. Alcohol policy in Europe: evidence from AMPHORA. The AMPHORA project. 2nd edn 2013; Chapter 9, pp 87–92. http://www.amphoraproject.net
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous