Neurofilament light polypeptide gene N98S mutation in mice leads to neurofilament network abnormalities and a Charcot-Marie-Tooth Type 2E phenotype
- PMID: 25552649
- PMCID: PMC4380066
- DOI: 10.1093/hmg/ddu736
Neurofilament light polypeptide gene N98S mutation in mice leads to neurofilament network abnormalities and a Charcot-Marie-Tooth Type 2E phenotype
Abstract
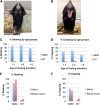
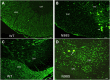
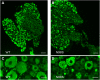
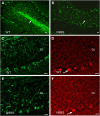
Charcot-Marie-Tooth disease (CMT) is the most commonly inherited neurological disorder with a prevalence of 1 in 2500 people worldwide. Patients suffer from degeneration of the peripheral nerves that control sensory information of the foot/leg and hand/arm. Multiple mutations in the neurofilament light polypeptide gene, NEFL, cause CMT2E. Previous studies in transfected cells showed that expression of disease-associated neurofilament light chain variants results in abnormal intermediate filament networks associated with defects in axonal transport. We have now generated knock-in mice with two different point mutations in Nefl: P8R that has been reported in multiple families with variable age of onset and N98S that has been described as an early-onset, sporadic mutation in multiple individuals. Nefl(P8R/+) and Nefl(P8R/P8R) mice were indistinguishable from Nefl(+/+) in terms of behavioral phenotype. In contrast, Nefl(N98S/+) mice had a noticeable tremor, and most animals showed a hindlimb clasping phenotype. Immunohistochemical analysis revealed multiple inclusions in the cell bodies and proximal axons of spinal cord neurons, disorganized processes in the cerebellum and abnormal processes in the cerebral cortex and pons. Abnormal processes were observed as early as post-natal day 7. Electron microscopic analysis of sciatic nerves showed a reduction in the number of neurofilaments, an increase in the number of microtubules and a decrease in the axonal diameters. The Nefl(N98S/+) mice provide an excellent model to study the pathogenesis of CMT2E and should prove useful for testing potential therapies.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures




References
-
- Shy M.E., Patzko A. (2011) Axonal Charcot-Marie-Tooth disease. Curr. Opin. Neurol., 24, 475–483. - PubMed
-
- Suter U., Scherer S.S. (2003) Disease mechanisms in inherited neuropathies. Nat. Rev. Neurosci., 4, 714–726. - PubMed
-
- Dyck P.J., Lambert E.H. (1968) Lower motor and primary sensory neuron diseases with peroneal muscular atrophy. II. Neurologic, genetic, and electrophysiologic findings in various neuronal degenerations. Arch. Neurol., 18, 619–625. - PubMed
-
- Dyck P.J., Lambert E.H. (1968) Lower motor and primary sensory neuron diseases with peroneal muscular atrophy. I. Neurologic, genetic, and electrophysiologic findings in hereditary polyneuropathies. Arch. Neurol., 18, 603–618. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

