Nephrocystin proteins NPHP5 and Cep290 regulate BBSome integrity, ciliary trafficking and cargo delivery
- PMID: 25552655
- PMCID: PMC4380068
- DOI: 10.1093/hmg/ddu738
Nephrocystin proteins NPHP5 and Cep290 regulate BBSome integrity, ciliary trafficking and cargo delivery
Abstract
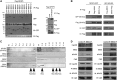
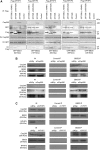
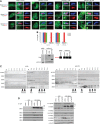
Proper functioning of cilia, hair-like structures responsible for sensation and locomotion, requires nephrocystin-5 (NPHP5) and a multi-subunit complex called the Bardet-Biedl syndrome (BBS)ome, but their precise relationship is not understood. The BBSome is involved in the trafficking of membrane cargos to cilia. While it is known that a loss of any single subunit prevents ciliary trafficking of the BBSome and its cargos, the mechanisms underlying ciliary entry of this complex are not well characterized. Here, we report that a transition zone protein NPHP5 contains two separate BBS-binding sites and interacts with the BBSome to mediate its integrity. Depletion of NPHP5, or expression of NPHP5 mutant missing one binding site, specifically leads to dissociation of BBS2 and BBS5 from the BBSome and loss of ciliary BBS2 and BBS5 without compromising the ability of the other subunits to traffic into cilia. Depletion of Cep290, another transition zone protein that directly binds to NPHP5, causes additional dissociation of BBS8 and loss of ciliary BBS8. Furthermore, delivery of BBSome cargos, smoothened, VPAC2 and Rab8a, to the ciliary compartment is completely disabled in the absence of single BBS subunits, but is selectively impaired in the absence of NPHP5 or Cep290. These findings define a new role of NPHP5 and Cep290 in controlling integrity and ciliary trafficking of the BBSome, which in turn impinge on the delivery of ciliary cargo.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures





Similar articles
-
BBS1 is involved in retrograde trafficking of ciliary GPCRs in the context of the BBSome complex.PLoS One. 2018 Mar 28;13(3):e0195005. doi: 10.1371/journal.pone.0195005. eCollection 2018. PLoS One. 2018. PMID: 29590217 Free PMC article.
-
A novel protein LZTFL1 regulates ciliary trafficking of the BBSome and Smoothened.PLoS Genet. 2011 Nov;7(11):e1002358. doi: 10.1371/journal.pgen.1002358. Epub 2011 Nov 3. PLoS Genet. 2011. PMID: 22072986 Free PMC article.
-
BBS7 is required for BBSome formation and its absence in mice results in Bardet-Biedl syndrome phenotypes and selective abnormalities in membrane protein trafficking.J Cell Sci. 2013 Jun 1;126(Pt 11):2372-80. doi: 10.1242/jcs.111740. Epub 2013 Apr 9. J Cell Sci. 2013. PMID: 23572516 Free PMC article.
-
Trafficking of ciliary membrane proteins by the intraflagellar transport/BBSome machinery.Essays Biochem. 2018 Dec 7;62(6):753-763. doi: 10.1042/EBC20180030. Print 2018 Dec 7. Essays Biochem. 2018. PMID: 30287585 Free PMC article. Review.
-
Ciliary protein trafficking mediated by IFT and BBSome complexes with the aid of kinesin-2 and dynein-2 motors.J Biochem. 2018 Mar 1;163(3):155-164. doi: 10.1093/jb/mvx087. J Biochem. 2018. PMID: 29272450 Review.
Cited by
-
A case of Bardet-Biedl syndrome caused by a recurrent variant in BBS12: A case report.Biomed Rep. 2021 Dec;15(6):103. doi: 10.3892/br.2021.1479. Epub 2021 Oct 21. Biomed Rep. 2021. PMID: 34760276 Free PMC article.
-
The Ciliary Transition Zone: Finding the Pieces and Assembling the Gate.Mol Cells. 2017 Apr;40(4):243-253. doi: 10.14348/molcells.2017.0054. Epub 2017 Apr 12. Mol Cells. 2017. PMID: 28401750 Free PMC article. Review.
-
Genetic testing including targeted gene panel in a diverse clinical population of children with autism spectrum disorder: Findings and implications.Mol Genet Genomic Med. 2018 Mar;6(2):171-185. doi: 10.1002/mgg3.354. Epub 2017 Dec 21. Mol Genet Genomic Med. 2018. PMID: 29271092 Free PMC article.
-
In vitro modeling and rescue of ciliopathy associated with IQCB1/NPHP5 mutations using patient-derived cells.Stem Cell Reports. 2022 Oct 11;17(10):2172-2186. doi: 10.1016/j.stemcr.2022.08.006. Epub 2022 Sep 8. Stem Cell Reports. 2022. PMID: 36084637 Free PMC article.
-
Alternative Splicing Shapes the Phenotype of a Mutation in BBS8 To Cause Nonsyndromic Retinitis Pigmentosa.Mol Cell Biol. 2015 May;35(10):1860-70. doi: 10.1128/MCB.00040-15. Epub 2015 Mar 16. Mol Cell Biol. 2015. PMID: 25776555 Free PMC article.
References
-
- Bornens M. (2012) The centrosome in cells and organisms. Science, 335, 422–426. - PubMed
-
- Hossain D., Tsang W.Y. (2013) Centrosome dysfunction and senescence: coincidence or causality? Aging Sci., 1, 113.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

