Allogeneic cell therapy: a new paradigm in therapeutics
- PMID: 25552688
- PMCID: PMC4411634
- DOI: 10.1161/CIRCRESAHA.114.305495
Allogeneic cell therapy: a new paradigm in therapeutics
Keywords: Editorials; allogeneic transplantation; autologous transplants; meta-analysis; stem cells.
Figures

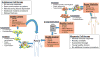
Comment on
-
Similar effect of autologous and allogeneic cell therapy for ischemic heart disease: systematic review and meta-analysis of large animal studies.Circ Res. 2015 Jan 2;116(1):80-6. doi: 10.1161/CIRCRESAHA.116.304872. Epub 2014 Sep 3. Circ Res. 2015. PMID: 25186794
References
-
- Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. - PMC - PubMed
-
- Houtgraaf JH, den Dekker WK, van Dalen BM, Springeling T, de Jong R, van Geuns RJ, Geleijnse ML, Fernandez-Aviles F, Zijlsta F, Serruys PW, Duckers HJ. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with st-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59:539–540. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

