Origin of cardiomyocytes in the adult heart
- PMID: 25552694
- PMCID: PMC4283577
- DOI: 10.1161/CIRCRESAHA.116.303595
Origin of cardiomyocytes in the adult heart
Erratum in
-
Correction.Circ Res. 2015 Dec 4;117(12):e133. doi: 10.1161/RES.0000000000000081. Circ Res. 2015. PMID: 26635387 No abstract available.
Abstract
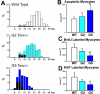
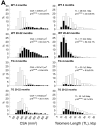
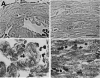
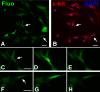
This review article discusses the mechanisms of cardiomyogenesis in the adult heart. They include the re-entry of cardiomyocytes into the cell cycle; dedifferentiation of pre-existing cardiomyocytes, which assume an immature replicating cell phenotype; transdifferentiation of hematopoietic stem cells into cardiomyocytes; and cardiomyocytes derived from activation and lineage specification of resident cardiac stem cells. The recognition of the origin of cardiomyocytes is of critical importance for the development of strategies capable of enhancing the growth response of the myocardium; in fact, cell therapy for the decompensated heart has to be based on the acquisition of this fundamental biological knowledge.
Keywords: cell dedifferentiation; cell transdifferentiation; stem cells.
© 2015 American Heart Association, Inc.
Figures







Similar articles
-
Cardiomyocytes re-enter the cell cycle and contribute to heart development after differentiation from cardiac progenitors expressing Isl1 in chick embryo.Dev Growth Differ. 2007 Apr;49(3):229-39. doi: 10.1111/j.1440-169X.2007.00923.x. Dev Growth Differ. 2007. PMID: 17394601
-
Continuous engraftment and differentiation of male recipient Y-chromosome-positive cardiomyocytes in donor female human heart transplants.J Heart Lung Transplant. 2007 Nov;26(11):1110-8. doi: 10.1016/j.healun.2007.08.004. J Heart Lung Transplant. 2007. PMID: 18022076
-
Role of D-type cyclins in heart development and disease.Can J Physiol Pharmacol. 2012 Sep;90(9):1197-207. doi: 10.1139/y2012-037. Epub 2012 Aug 17. Can J Physiol Pharmacol. 2012. PMID: 22900666 Review.
-
Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes as a Model for Heart Development and Congenital Heart Disease.Stem Cell Rev Rep. 2015 Oct;11(5):710-27. doi: 10.1007/s12015-015-9596-6. Stem Cell Rev Rep. 2015. PMID: 26085192 Free PMC article. Review.
-
How to Stimulate Myocardial Regeneration in Adult Mammalian Heart: Existing Views and New Approaches.Biomed Res Int. 2020 Mar 3;2020:7874109. doi: 10.1155/2020/7874109. eCollection 2020. Biomed Res Int. 2020. PMID: 32190680 Free PMC article. Review.
Cited by
-
Identification of a multipotent Twist2-expressing cell population in the adult heart.Proc Natl Acad Sci U S A. 2018 Sep 4;115(36):E8430-E8439. doi: 10.1073/pnas.1800526115. Epub 2018 Aug 20. Proc Natl Acad Sci U S A. 2018. PMID: 30127033 Free PMC article.
-
Pharmacological Therapy in the Heart as an Alternative to Cellular Therapy: A Place for the Brain Natriuretic Peptide?Stem Cells Int. 2016;2016:5961342. doi: 10.1155/2016/5961342. Epub 2016 Jan 4. Stem Cells Int. 2016. PMID: 26880973 Free PMC article. Review.
-
A comprehensive review on 3D tissue models: Biofabrication technologies and preclinical applications.Biomaterials. 2024 Jan;304:122408. doi: 10.1016/j.biomaterials.2023.122408. Epub 2023 Nov 27. Biomaterials. 2024. PMID: 38041911 Free PMC article. Review.
-
Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease.Nat Rev Drug Discov. 2016 Sep;15(9):620-638. doi: 10.1038/nrd.2016.89. Epub 2016 Jun 24. Nat Rev Drug Discov. 2016. PMID: 27339799 Free PMC article. Review.
-
Extracellular vesicles in cardiac repair and regeneration: Beyond stem-cell-based approaches.Front Cell Dev Biol. 2022 Sep 2;10:996887. doi: 10.3389/fcell.2022.996887. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36120584 Free PMC article. Review.
References
-
- Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res. 1998;83:1–1. - PubMed
-
- Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res. 1998;83:15–15. - PubMed
-
- Braun T, Martire A. Cardiac stem cells: paradigm shift or broken promise? A view from developmental biology. Trends Biotechnol. 2007;25:441–441. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AG017042/AG/NIA NIH HHS/United States
- R01 HL111183/HL/NHLBI NIH HHS/United States
- P01 AG043353/AG/NIA NIH HHS/United States
- R01 HL114346/HL/NHLBI NIH HHS/United States
- P01 HL092868/HL/NHLBI NIH HHS/United States
- R01 HL091021/HL/NHLBI NIH HHS/United States
- R01 HL039902/HL/NHLBI NIH HHS/United States
- R01 HL105532/HL/NHLBI NIH HHS/United States
- R01 AG037495/AG/NIA NIH HHS/United States
- R01 AG026107/AG/NIA NIH HHS/United States
- R01 HL065577/HL/NHLBI NIH HHS/United States
- R37 HL081737/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

